Curcumin
| Code | Size | Price |
|---|
| CDX-C0158-G010 | 10 g | £59.00 |
Quantity:
| CDX-C0158-G050 | 50 g | £169.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Host Type: Plant
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
Short Term: +4°C, Long Term: -20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
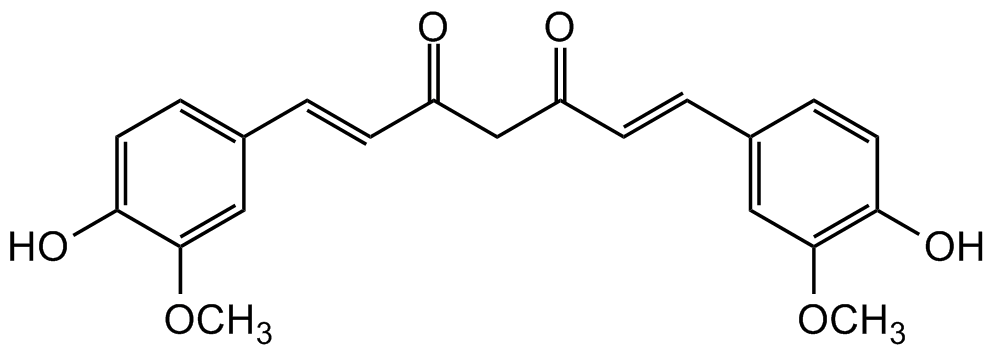
(E,E)-1,7-bis(4-Hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione; Diferuloylmethane; Diferulylmethane; Diferuloylmethane; Natural Yellow 3
Appearance:
Yellow to orange solid.
CAS:
458-37-7
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C21H20O6/c1-26-20-11-14(5-9-18(20)24)3-7-16(22)13-17(23)8-4-15-6-10-19(25)21(12-15)27-2/h3-12,24-25H,13H2,1-2H3/b7-3+,8-4+
InChiKey:
VFLDPWHFBUODDF-FCXRPNKRSA-N
Long Description:
Chemical. CAS: 458-37-7. Formula: C21H20O6. MW: 368.38. Curcumin is the major yellow pigment in turmeric and curry and has antioxidant, anti-inflammatory, neuroprotective, cardioprotective, antidiabetic, antiviral, antibacterial, antifungal, chemopreventive and antitumor activities. It interferes with multiple cell signaling pathways, including cell cycle (cyclin D1 and cyclin E), apoptosis (activation of caspases and down-regulation of anti-apoptotic gene products), proliferation (HER-2, EGFR and AP-1), survival (PI3K/AKT pathway), invasion (MMP-9 and adhesion molecules), angiogenesis (VEGF), metastasis (CXCR-4), tumorigenesis and development (Shh, Gli), metabolism (PPARgamma, Nrf2), epigenetics (HDACs, HATs, DNA methyltransferase I and microRNAs) and inflammation (NLRP3, TLR4, NF-kappaB, TNF, IL-6, IL-1, COX-2 and 5-LOX).
MDL:
MFCD00008365
Molecular Formula:
C21H20O6
Molecular Weight:
368.38
Package Type:
Vial
Product Description:
Curcumin is the major yellow pigment in turmeric and curry and has antioxidant, anti-inflammatory, neuroprotective, cardioprotective, antidiabetic, antiviral, antibacterial, antifungal, chemopreventive and antitumor activities. It interferes with multiple cell signaling pathways, including cell cycle (cyclin D1 and cyclin E), apoptosis (activation of caspases and down-regulation of anti-apoptotic gene products), proliferation (HER-2, EGFR and AP-1), survival (PI3K/AKT pathway), invasion (MMP-9 and adhesion molecules), angiogenesis (VEGF), metastasis (CXCR-4), tumorigenesis and development (Shh, Gli), metabolism (PPARgamma, Nrf2), epigenetics (HDACs, HATs, DNA methyltransferase I and microRNAs) and inflammation (NLRP3, TLR4, NF-kappaB, TNF, IL-6, IL-1, COX-2 and 5-LOX).
Purity:
>95%
SMILES:
OC1=CC=C(/C=C/C(CC(/C=C/C2=CC(OC)=C(O)C=C2)=O)=O)C=C1OC
Solubility Chemicals:
Soluble in DMSO (25mg/ml) or ethanol (10mg/ml).
Source / Host:
Plant
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) R.K. Maheshwari, et al.; Life Sci. 78, 2081 (2006) (Review) | (2) V.P. Menon & A.R. Sudheer; Adv. Exp. Med. Biol. 595, 105 (2007) (Review) | (3) G. Kuttan, et al.; Adv. Exp. Med. Biol. 595, 173 (2007) (Review) | (4) B.B. Aggarwal & K.B. Harikumar; Int. J. Biochem. Cell Biol. 41, 40 (2009) (Review) | (5) S. Fu & R. Kurzrock; Cancer 116, 4670 (2010) (Review) | (6) L. Alappat & A.B. Awad; Nutr. Rev. 68, 729 (2010) (Review) | (7) R.B. Mythri & M.M. Bharath; Curr. Pharm. Des. 18, 91 (2012) (Review) | (8) A.A. Momtazi, et al.; Rev. Physiol. Biochem. Pharmacol. 171, 1 (2016) (Review) | (9) S.S. Soflaei, et al.; Curr. Pharm. Des. 24, 123 (2018) (Review) | (10) M. Boozari, et al.; J. Cell Physiol. 234, 12471 (2019) (Review) | (11) K.R. Kahkhaie, et al.; Inflammopharmacol. 27, 885 (2019) (Review) | (12) D.J.Den Hartogh, et al.; Nutrients 12, 118 (2020) (Review) | (13) D.J.Den Hartogh, et al.; Nutrients 12, 58 (2020) (Review) | (14) M.A. Panaro, et al.; Int. J. Mol. Sci. 21, 2299 (2020) (Review) | (15) M.R. Jennings & R.J. Parks; Viruses 12, 1242 (2020) (Review) | (16) A.Saeedi-Boroujeni, et al.; Basic. Clin. Pharmacol. Toxicol. 128, 37 (2021) (Review)