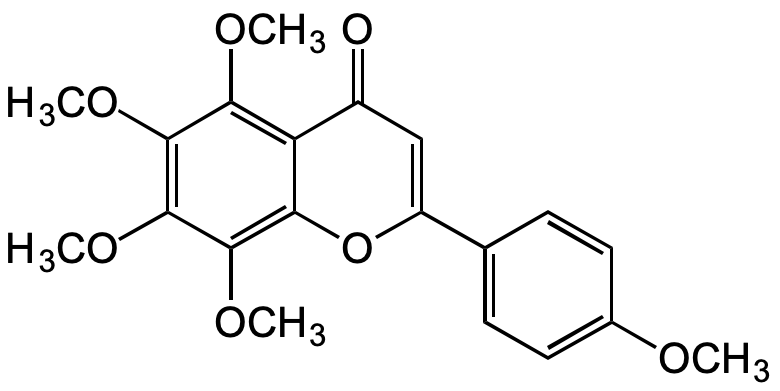
Tangeretin
| Code | Size | Price |
|---|
| CDX-T0415-M005 | 5 mg | £59.00 |
Quantity:
| CDX-T0415-M010 | 10 mg | £96.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Host Type: Plant
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
+20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
4',5,6,7,8-Pentamethoxyflavone; NSC 53909; NSC 618905; Ponkanetin; 5,6,7,8-Tetramethoxy-2-(4-methoxyphenyl)-4H-1-benzopyran-4-one
Appearance:
White to pale yellow powder.
CAS:
481-53-8
Class:
6.1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS06
Handling Advice:
Protect from light and moisture.
Hazards:
H300
InChi:
InChI=1S/C20H20O7/c1-22-12-8-6-11(7-9-12)14-10-13(21)15-16(23-2)18(24-3)20(26-5)19(25-4)17(15)27-14/h6-10H,1-5H3
InChiKey:
ULSUXBXHSYSGDT-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 481-53-8. Formula: C20H20O7. MW: 372.37. Isolated from plant source. Tangeritin is a polymethoxylated flavone isolated from peels of citrus fruits with antiproliferative and antioxidant properties. It inhibits signaling in cancer cells, reducing ERK phosphorylation and growth of estradiol-stimulated T47D breast cancer cells and blocking p38 MAPK, JNK, and Akt activation in interleukin-1beta-stimulated human lung carcinoma A549 cells. It is described to block cell cycle progression at G1 and induce apoptosis. Shows anti-inflammatory, osteoprotective and neuroprotective effects. Tangeritin activates the pregnane X receptor, inducing MDR1 expression in human colonic LS180 cancer cells. Shown to be a Notch-1 inhibitor. Tangeretin stimulates glucose uptake via regulation of AMPK signaling pathways and has been shown to antagonize ABCB1-mediated multidrug resistance.
MDL:
MFCD00017438
Molecular Formula:
C20H20O7
Molecular Weight:
372.37
Package Type:
Vial
PG:
III
Precautions:
P264-P301 + P310
Product Description:
Tangeritin is a polymethoxylated flavone isolated from peels of citrus fruits with antiproliferative and antioxidant properties. It inhibits signaling in cancer cells, reducing ERK phosphorylation and growth of estradiol-stimulated T47D breast cancer cells and blocking p38 MAPK, JNK, and Akt activation in interleukin-1beta-stimulated human lung carcinoma A549 cells. It is described to block cell cycle progression at G1 and induce apoptosis. Shows anti-inflammatory, osteoprotective and neuroprotective effects. Tangeritin activates the pregnane X receptor, inducing MDR1 expression in human colonic LS180 cancer cells. Shown to be a Notch-1 inhibitor. Tangeretin stimulates glucose uptake via regulation of AMPK signaling pathways and has been shown to antagonize ABCB1-mediated multidrug resistance.
Purity:
>98% (HPLC)
Signal word:
Danger
SMILES:
O=C1C=C(C2=CC=C(OC)C=C2)OC3=C(OC)C(OC)=C(OC)C(OC)=C31
Solubility Chemicals:
Soluble in DMSO (10mg/ml) or DMF (10mg/ml). Sparingly soluble in ethanol (0,5 mg/ml).
Source / Host:
Isolated from plant source.
Transportation:
Excepted Quantity
UN Nummer:
2811
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at RT.
References
(1) T. Hirano, et al.; Br. J. Cancer 72, 1380 (1995) | (2) C. Chaumontet, et al.; Cancer Lett. 114, 207 (1997) | (3) S. Kawaii, et al.; Biosci. Biotechnol. Biochem. 63, 896 (1999) | (4) K.P. Datla, et al.; Neuroreport 12, 3871 (2001) | (5) M.H. Pan, et al.; Carcinogenesis 23, 1677 (2002) | (6) S. Van Slambrouck, et al.; FEBS Lett. 579, 1665 (2005) | (7) K.H. Chen, et al.; Biochem. Pharmacol. 73, 215 (2007) | (8) K.L. Morley, et al.; Cancer Lett. 251, 168 (2007) | (9) H. Satsu, et al.; J. Agricult. Food Chem. 56, 5366 (2008) | (10) M.S. Kim, et al.; Mol. Cell Endocrinol. 358, 127 (2012) | (11) Z. Shu, et al.; Int. Immunopharmacol. 19, 275 (2014) | (12) Y.Y. Lee, et al.; J. Neuroimmune Pharmacol. 11, 294 (2016) | (13) S.L. Feng, et al.; Pharmacol. Res. 110, 193 (2016) | (14) L.L. Ma, et al.; Biomed. Pharmacother. 81, 491 (2016) | (15) N. Braidy, et al.; CNS Neurol. Disord. Drug Targets 16, 387 (2017) (Review) | (16) S. Xu, et al.; Int. Immunopharmacol. 72, 402 (2019)