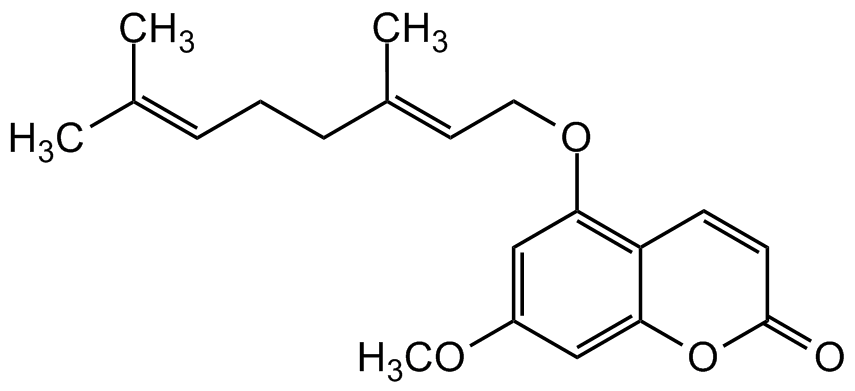
5-Geranyloxy-7-methoxycoumarin
| Code | Size | Price |
|---|
| CDX-G0228-M001 | 1 mg | £84.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Host Type: Plant
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
Short Term: +4°C Long Term: -20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
5-G-7-MOC; 7-Methoxy-5-geranoxycoumarin
Appearance:
White to off-white powder.
CAS:
7380-39-4
EClass:
32160000
Form (Short):
solid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C20H24O4/c1-14(2)6-5-7-15(3)10-11-23-18-12-16(22-4)13-19-17(18)8-9-20(21)24-19/h6,8-10,12-13H,5,7,11H2,1-4H3/b15-10+
InChiKey:
WXUOSNJWDJOHGW-XNTDXEJSSA-N
Long Description:
Chemical. CAS: 7380-39-4. Formula: C20H24O4. MW: 328.4. 5-Geranyloxy-7-methoxycoumarin is a natural coumarin found in the essential oils of citrus such as bergamot, which shows antifungal, antibacterial, and anticancer activity. 5-Geranyloxy-7-methoxycoumarin arrests cells at the G0/G1 phase and induces apoptosis through activation of tumour suppressor gene p53, caspase8/3, regulation of Bcl2 and inhibition of p38 MAPK phosphorylation.
MDL:
MFCD00210506
Molecular Formula:
C20H24O4
Molecular Weight:
328.4
Package Type:
Vial
Product Description:
5-Geranyloxy-7-methoxycoumarin is a natural coumarin found in the essential oils of citrus such as bergamot, which shows antifungal, antibacterial, and anticancer activity. 5-Geranyloxy-7-methoxycoumarin arrests cells at the G0/G1 phase and induces apoptosis through activation of tumour suppressor gene p53, caspase8/3, regulation of Bcl2 and inhibition of p38 MAPK phosphorylation.
Purity:
>98% (HPLC)
SMILES:
O=C1OC2=CC(OC)=CC(OC/C=C(CC/C=C(C)C)C)=C2C=C1
Solubility Chemicals:
Soluble in DMSO or chloroform.
Source / Host:
Isolated from plant source.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) M. Benincasa, et al.; Chromatographia 30, 271 (1990) | (2) S. Ben-Yehoshua, et al.; J. Agric. Food Chem. 40, 1217 (1992) | (3) Y. Miyake, et al.; J. Agric. Food Chem. 47, 3151 (1999) | (4) Y. Miyake & M. Hiramitsu; J. Food Sci. Technol. 48, 635 (2011) | (5) J.R. Patil, et al.; Planta Med. 79, 219 (2013) | (6) G.Y. Zuo, et al.; Phytomedicine 23, 1814 (2016) | (7) C. Ramirez-Pelayo, et al.; Heliyon 5, e01937 (2019) | (8) A. Maugeri, et al.; Toxins 13, 275 (2021)