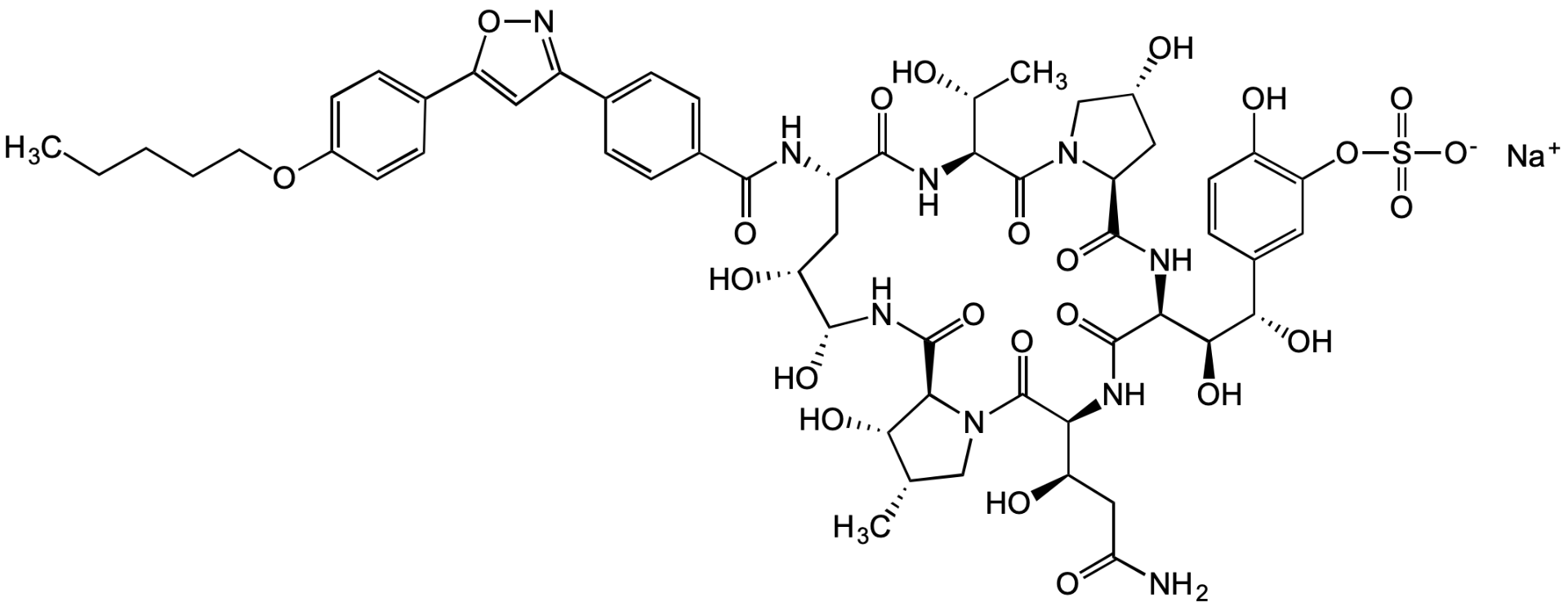
Micafungin sodium
| Code | Size | Price |
|---|
| CDX-M0187-M005 | 5 mg | £78.00 |
Quantity:
| CDX-M0187-M025 | 25 mg | £280.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
BLUE ICE
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Mycamine; Funguard; FK463; 1-[(4R,5R)-4,5-Dihydroxy-N2-[4-[5-[4-(pentyloxy)phenyl]-3-isoxazolyl]benzoyl]-L-ornithine]-4-[(4S)-4-hydroxy-4-[4-hydroxy-3-(sulfooxy)phenyl]-L-threonine]pneumocandin A0 sodium salt
Appearance:
White to beige powder.
CAS:
208538-73-2
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C56H71N9O23S.Na/c1-4-5-6-17-86-32-14-11-28(12-15-32)39-21-33(63-87-39)27-7-9-29(10-8-27)49(75)58-34-20-38(70)52(78)62-54(80)45-46(72)25(2)23-65(45)56(82)43(37(69)22-41(57)71)60-53(79)44(48(74)47(73)30-13-16-36(68)40(18-30)88-89(83,84)85)61-51(77)35-19-31(67)24-64(35)55(81)42(26(3)66)59-50(34)76;/h7-16,18,21,25-26,31,34-35,37-38,42-48,52,66-70,72-74,78H,4-6,17,19-20,22-24H2,1-3H3,(H2,57,71)(H,58,75)(H,59,76)(H,60,79)(H,61,77)(H,62,80)(H,83,84,85);/q;+1/p-1/t25-,26+,31+,34-,35-,37+,38+,42-,43-,44-,45-,46-,47-,48-,52+;/m0./s1
InChiKey:
KOOAFHGJVIVFMZ-WZPXRXMFSA-M
Long Description:
Chemical. CAS: 208538-73-2. Formula: C56H70N9NaO23S. MW: 1292.26. Semisynthetic. Micafungin is a semisynthetic echinocandin antifungal. Micafungin inhibits the enzyme beta(1,3)-D-Glucan synthase, consequently 1,3-beta-D-glucan synthesis and thereby disturbing the integrity of the fungal cell wall. It has fungicidal activity against virtually all species of Candida, including those resistant to fluconazole, and is fungistatic against Aspergillus spp. The target enzyme does not exist in mammalian systems.
MDL:
MFCD08067752
Molecular Formula:
C56H70N9NaO23S
Molecular Weight:
1292.26
Package Type:
Vial
Product Description:
Micafungin is a semisynthetic echinocandin antifungal. Micafungin inhibits the enzyme beta(1,3)-D-Glucan synthase, consequently 1,3-beta-D-glucan synthesis and thereby disturbing the integrity of the fungal cell wall. It has fungicidal activity against virtually all species of Candida, including those resistant to fluconazole, and is fungistatic against Aspergillus spp. The target enzyme does not exist in mammalian systems.
Purity:
>97% (HPLC)
SMILES:
O[C@@H]([C@@H](C)C1)[C@@H](C(N[C@@H]([C@@H](C2)O)O)=O)N1C([C@H]([C@@H](CC(N)=O)O)NC([C@H]([C@@H]([C@H](C(C=C3)=CC(OS([O-])(=O)=O)=C3O)O)O)NC([C@H](C[C@H]4O)N(C4)C([C@H]([C@@H](C)O)NC([C@H]2NC(C(C=C5)=CC=C5C6=NOC(C(C=C7)=CC=C7OCCCCC)=C6)=O)=O)=O)=O)=O)=O.[Na+]
Solubility Chemicals:
Soluble in DMSO or DMF (both 10mg/ml). Slightly soluble in ethanol, methanol or water (all 1mg/ml).
Source / Host:
Semisynthetic
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) M. Tomishima, et al.; J. Antibiot. 52, 674 (1999) | (2) R.A. Fromtling; Drugs Today 38, 245 (2002) (Review) | (3) A.J. Carillo-Munoz, et al.; Rev. Esp. Quimioter. 19, 130 (2006) | (4) J.J. Vehreschild & O.A. Cornely; Future Microbiol. 1, 161 (2006) | (5) J.M. Joseph, et al.; Pharmacotherapy 27, 53 (2007) (Review) | (6) N.P. Wiederhold & J.S. Lewis; Expert Opin. Pharmacother. 8, 1155 (2007) (Review) | (7) A.M. Bormann & V.A. Morrison; Drug. Des. Devel. Ther. 3, 295 (2009)