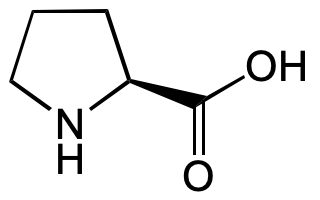
L-Proline
| Code | Size | Price |
|---|
| CDX-P0446-G100 | 100 g | £102.00 |
Quantity:
| CDX-P0446-K001 | 1 kg | £560.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
+20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
(S)-Pyrrolidine-2-carboxylic acid; H-Pro-OH
Appearance:
White to off-white powder.
CAS:
147-85-3
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C5H9NO2/c7-5(8)4-2-1-3-6-4/h4,6H,1-3H2,(H,7,8)/t4-/m0/s1
InChiKey:
ONIBWKKTOPOVIA-BYPYZUCNSA-N
Long Description:
Chemical. CAS: 147-85-3. Formula: C5H9NO2. MW: 115.13. Synthetic. Proline is a proteinogenic amino acid that is used in the biosynthesis of proteins. L-Proline has been found to act as a weak agonist of the glycine receptor and of both NMDA and non-NMDA (AMPA/kainate) ionotropic glutamate receptors. L-proline is essential for induction of hepatocyte proliferation in culture, through its affect on synthesis of intracellular collagen. It has free radical scavenging potential. Induces differentiation of embyonic stem cells and has been studied as regulator of pluripotent cells in culture. Shows cryoprotective properties. L-Proline is used as asymmetric catalysts in organic synthesis and asymmetric aldol cyclization. It is involved in the Michael addition of dimethyl malonate to alfa-beta-unsaturated aldehydes. It finds uses in pharmaceutical, biotechnological applications due to its osmoprotectant property.
MDL:
MFCD00064318
Molecular Formula:
C5H9NO2
Molecular Weight:
115.13
Package Type:
Vial
Product Description:
Proline is a proteinogenic amino acid that is used in the biosynthesis of proteins. L-Proline has been found to act as a weak agonist of the glycine receptor and of both NMDA and non-NMDA (AMPA/kainate) ionotropic glutamate receptors. L-proline is essential for induction of hepatocyte proliferation in culture, through its affect on synthesis of intracellular collagen. It has free radical scavenging potential. Induces differentiation of embyonic stem cells and has been studied as regulator of pluripotent cells in culture. Shows cryoprotective properties. L-Proline is used as asymmetric catalysts in organic synthesis and asymmetric aldol cyclization. It is involved in the Michael addition of dimethyl malonate to alfa-beta-unsaturated aldehydes. It finds uses in pharmaceutical, biotechnological applications due to its osmoprotectant property.
Purity:
>98% (HPLC)
SMILES:
O=C(O)[C@H]1NCCC1
Solubility Chemicals:
Soluble in water (50 mg/ml) or ethanol.
Source / Host:
Synthetic
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at RT.
References
(1) A. Van Harreveld & F. Strumwasser F.; Neuroscience 6, 2495 (1981) | (2) E. Keller, et al.; J. Neurochem. 37, 1335 (1981) | (3) T. Nakamura, et al.; BBRC 122, 884 (1984) | (4) V. Henzi, et al.; Mol. Pharmacol. 41, 793 (1992) | (5) S. Usse, et al.; J. Org. Chem. 65, 914 (2000) | (6) S. Kaul, et al.; Amino Acids 34, 315 (2008) | (7) N. Verbruggen & C. Hermans; Amino Acids 35, 753 (2008) (Review) | (8) J.M. Washington, et al.; Am. J. Physiol. Cell Physiol. 298, C982 (2010) | (9) G. Zhang, et al.; Chem. Commun. 49, 7908 (2013) | (10) S. Comes, et l.; Stem Cell Reports 1, 307 (2013) | (11) H. Jin, et al.; J. Org. CHem. 81, 3263 (2016) | (12) L. Zahng, et al.; Sci. Rep. 6, 26326 (2016)