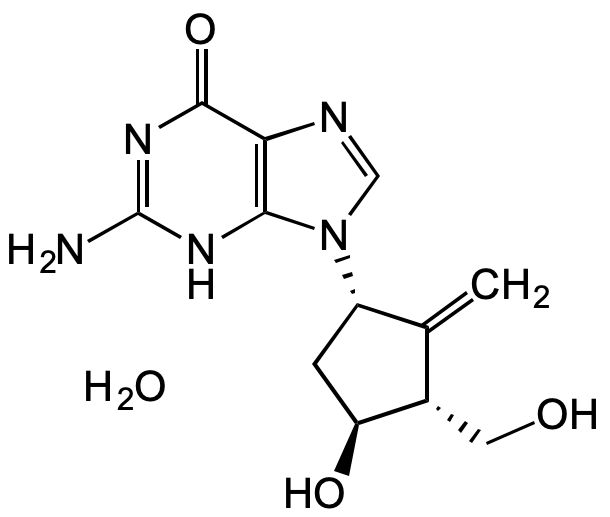
Entecavir monohydrate
| Code | Size | Price |
|---|
| CDX-E0229-M050 | 50 mg | £96.00 |
Quantity:
| CDX-E0229-M200 | 200 mg | £267.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
+20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Baraclude; BMS 200475; SQ 34,676; 2-Amino-1,9-dihydro-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]-6H-purin-6-one monohydrate
Appearance:
White to off-white powder.
CAS:
209216-23-9
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C12H15N5O3.H2O/c1-5-6(3-18)8(19)2-7(5)17-4-14-9-10(17)15-12(13)16-11(9)20;/h4,6-8,18-19H,1-3H2,(H3,13,15,16,20);1H2/t6-,7-,8-;/m0./s1
InChiKey:
YXPVEXCTPGULBZ-WQYNNSOESA-N
Long Description:
Chemical. CAS: 209216-23-9. Formula: C12H15N5O3 . H2O. MW: 295.29. Synthetic. Entecavir is an oral antiviral drug used in the treatment of hepatitis B (HBV) infection. It is marketed under the trade name Baraclude (BMS). Entecavir is a potent deoxyguanosine nucleoside analog with antiviral activity selective for hepadnaviruses. In vitro, the active intracellular form of entecavir, entecavir triphosphate, demonstrates a higher binding affinity for HBV DNA polymerase than the natural guanosine triphosphate substrate and effectively inhibits HBV DNA replication at 3 stages in the replication pathway: priming, reverse transcription and DNA-dependent DNA synthesis. Can be also used as a reference compound.
MDL:
MFCD09754448
Molecular Formula:
C12H15N5O3 . H2O
Molecular Weight:
295.29
Package Type:
Vial
Product Description:
Entecavir is an oral antiviral drug used in the treatment of hepatitis B (HBV) infection. It is marketed under the trade name Baraclude (BMS). Entecavir is a potent deoxyguanosine nucleoside analog with antiviral activity selective for hepadnaviruses. In vitro, the active intracellular form of entecavir, entecavir triphosphate, demonstrates a higher binding affinity for HBV DNA polymerase than the natural guanosine triphosphate substrate and effectively inhibits HBV DNA replication at 3 stages in the replication pathway: priming, reverse transcription and DNA-dependent DNA synthesis. Can be also used as a reference compound.
Purity:
>98% (HPLC)
SMILES:
O=C1C2=C(N([C@H]3C[C@H](O)[C@@H](CO)C3=C)C=N2)NC(N)=N1.O
Solubility Chemicals:
Solube in DMSO (10mg/ml), DMF (10mg/ml) or methanol. Sparingly soluble in water.
Source / Host:
Synthetic
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at RT.
References
(1) S.F. Innaimo, et al.; Antimicrob. Agents Chemother. 41, 1444 (1997) | (2) M. Seifer, et al.; Antimicrob. Agents Chemother. 42, 3200 (1998) | (3) P. Honkoop & R.A. De Man; Expert. Opin. Investig. Drugs 12, 683 (2003) (Review) | (4) S.J. Matthews; Clin. Ther. 28, 184 (2006) (Review) | (5) K.A. Sims & A.M. Woodland; Pharmacotherapy 26, 1745 (2006) (Review) | (6) P.N. Cheng & T.T. Chang; Expert Rev. Anti. Infect. Ther. 6, 569 (2008) (Review)