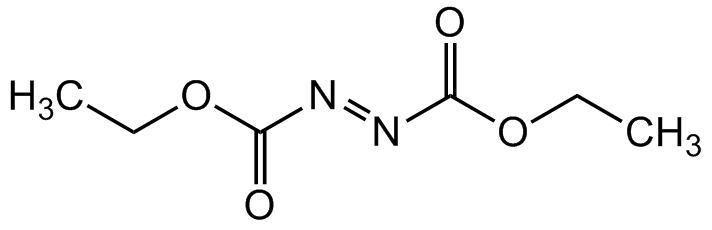
Diethyl azodicarboxylate
| Code | Size | Price |
|---|
| CDX-D0095-G010 | 10 g | £48.00 |
Quantity:
| CDX-D0095-G050 | 50 g | £157.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
+20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
DEAD; 1,2-Ethoxycarbonyl diazene; Diethoxycarbonyldiazene; NSC 3474; NSC 679015; Unifoam AZ-AE 200
Appearance:
Red clear liquid.
CAS:
1972-28-7
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C6H10N2O4/c1-3-11-5(9)7-8-6(10)12-4-2/h3-4H2,1-2H3/b8-7+
InChiKey:
FAMRKDQNMBBFBR-BQYQJAHWSA-N
Long Description:
Chemical. CAS: 1972-28-7. Formula: C6H10N2O4. MW: 174.15. Synthetic. Reagent for synthesis. Diethyl azodicarboxylate acts as a dienophile used in Diels-Alder reactions. It is used as a reagent in alpha-thiocyanation of enolizable ketones with ammonium thiocyanate and annulation of N-protected imines. It acts as a reactant for the preparation of immunostimulants alpha-Galactosylceramides, bisubstrate inhibitors with molecular recognition at the active site of catechol-O-methyltransferase and aza-beta-lactams via NHC-catalyzed [2 + 2] cycloaddition with ketenes. It is a commonly used activating reagent in Mitsunobu reaction and used in the synthesis of pharmaceuticals like zidovudine and procarbazine. In addition, it is an efficient dehydrogenating agent, which is involved in the preparation of aldehydes, disulfides, hydrazo groups from alcohols, thiols and azo goups, respectively. It is also a good electron acceptor. It is mostly known as a key component of the Mitsunobu reaction, a common strategy for the preparation of an amine, azide, ether, thioether or ester from the corresponding alcohol.
MDL:
MFCD00009103
Molecular Formula:
C6H10N2O4
Molecular Weight:
174.15
Package Type:
Vial
Product Description:
Reagent for synthesis. Diethyl azodicarboxylate acts as a dienophile used in Diels-Alder reactions. It is used as a reagent in alpha-thiocyanation of enolizable ketones with ammonium thiocyanate and annulation of N-protected imines. It acts as a reactant for the preparation of immunostimulants alpha-Galactosylceramides, bisubstrate inhibitors with molecular recognition at the active site of catechol-O-methyltransferase and aza-beta-lactams via NHC-catalyzed [2 + 2] cycloaddition with ketenes. It is a commonly used activating reagent in Mitsunobu reaction and used in the synthesis of pharmaceuticals like zidovudine and procarbazine. In addition, it is an efficient dehydrogenating agent, which is involved in the preparation of aldehydes, disulfides, hydrazo groups from alcohols, thiols and azo goups, respectively. It is also a good electron acceptor. It is mostly known as a key component of the Mitsunobu reaction, a common strategy for the preparation of an amine, azide, ether, thioether or ester from the corresponding alcohol.
Purity:
>98%
SMILES:
O=C(/N=N/C(OCC)=O)OCC
Solubility Chemicals:
Soluble in chloroform or DCM. Insoluble in water.
Source / Host:
Synthetic
Transportation:
Excepted Quantity
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at RT.
References
(1) F. Yoneda, et al.; JACS 88, 2328 (1966) | (2) E.C. Taylor & F. Yoneda; Chem. Commun. 1967, 199 (1967) | (3) E.E. Smissman & A. Makriyannis; J. Org. Chem. 38, 1652 (1973) | (4) L.A. Pauette; Encycl. Reag. Org. Synth. 3, 1790 (1995) | (5) M. Shi & G.-L. Zhao; Tetrahedr. 60, 2083 (2004) | (6) R. Dembinski; Eur. J. Org. Chem. 2004, 2763 (2004) | (7) V. Nair, et al.; Angew. Chem. Int. Ed. Engl. 46, 2070 (2007) | (8) E.J. Stoner & A.C. Hart; Encycl. Reag. Org. Synth. (2010)