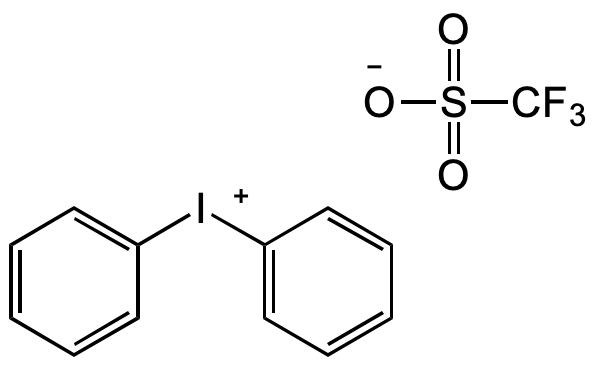
Diphenyliodonium triflate
| Code | Size | Price |
|---|
| CDX-D0091-G005 | 5 g | £248.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
+20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
DPIT; Diphenyliodonium trifluoromethanesulfonate
Appearance:
Light yellow crystalline.
CAS:
66003-76-7
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS07
Handling Advice:
Protect from light and moisture.
Hazards:
H315-H319-H335
InChi:
InChI=1S/C12H10I.CHF3O3S/c1-3-7-11(8-4-1)13-12-9-5-2-6-10-12;2-1(3,4)8(5,6)7/h1-10H;(H,5,6,7)/q+1;/p-1
InChiKey:
SBQIJPBUMNWUKN-UHFFFAOYSA-M
Long Description:
Chemical. CAS: 66003-76-7. Formula: C13H10F3IO3S. MW: 430.18. Synthetic. Reagent for synthesis. Used for alpha-phenylation of cyclic ketones, N-, O-, P-, and S-phenylation reactions. Phenylating agent for palladium-catalyzed cross-couplings, click chemistry and photochemistry.
MDL:
MFCD00191356
Molecular Formula:
C13H10F3IO3S
Molecular Weight:
430.18
Package Type:
Vial
Precautions:
P261-P305 + P351 + P338
Product Description:
Reagent for synthesis. Used for alpha-phenylation of cyclic ketones, N-, O-, P-, and S-phenylation reactions. Phenylating agent for palladium-catalyzed cross-couplings, click chemistry and photochemistry.
Purity:
>98% (NMR)
Signal Word:
Warning
SMILES:
[O-]S(=O)(C(F)(F)F)=O.C1([I+]C2=CC=CC=C2)=CC=CC=C1
Solubility Chemicals:
Soluble in acetone, chloroform, DMF or DMSO.
Source / Host:
Synthetic
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at RT.
References
(1) J.L. Dektar & N.P.; J. Org. Chem. 55, 639 (1990) | (2) J.H. Ryan & P.J. Stang; Tetrahed. Lett. 38, 5061 (1997) | (3) S.A. Jacobsen, et al.; J. Chem. Soc. Perkin Trans. I 1999, 3265 (1999) | (4) U. Radhakrishnan & P.J. Stang; Org. Lett. 3, 859 (2001) | (5) V.K. Aggarwal & B. Olofsson; Angew. Chem. Int. Ed. 44, 5516 (2005) | (6) J.-M. Becht & C. Drian; Org. Lett. 10, 3161 (2008) | (7) J. Aydin, et al.; Org. Lett. 11, 2852 (2009) | (8) D. Kumar & V.B. Reddy; Synthesis 10, 1687 (2010) | (9) D. Lapointe, et al.; Org. Chem. 76, 749 (2011) | (10) J.-M. Becht; Encycl. Reag. Org. Synth. (2011)