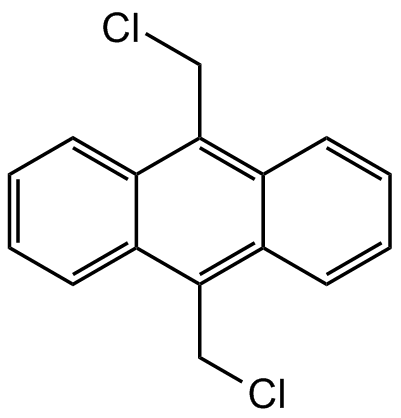
9,10-Bis(chloromethyl)anthracene
| Code | Size | Price |
|---|
| CDX-B0132-G005 | 5 g | £121.00 |
Quantity:
| CDX-B0132-G025 | 25 g | £450.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
+20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
NSC 81650; BCMA; ICR-450; BRN 2055024
Appearance:
Yellow powder.
CAS:
10387-13-0
Class:
8
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS05,GHS07
Handling Advice:
Protect from light and moisture.
Hazards:
H302, H314, H413
InChi:
InChI=1S/C16H12Cl2/c17-9-15-11-5-1-2-6-12(11)16(10-18)14-8-4-3-7-13(14)15/h1-8H,9-10H2
InChiKey:
UOSROERWQJTVNU-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 10387-13-0. Formula: C16H12CI2. MW: 275.18. Building block and intermediate for synthesis of fluorescent and luminescent agents. This compound has been used as a cross-linking agent to incorporate 9,10-anthracenylidene (AN) chromophores into linear polystyrene chains.
MDL:
MFCD00045388
Molecular Formula:
C16H12Cl2
Molecular Weight:
275.18
Package Type:
Vial
PG:
II
Precautions:
P260, P264, P270, P273, P280, P301 + P312, P301 + P330 + P331, P303 + P361 + P353, P304 + P340, P305 + P351 + P338, P310, P321, P363, P405, P501
Product Description:
Building block and intermediate for synthesis of fluorescent and luminescent agents. This compound has been used as a cross-linking agent to incorporate 9,10-anthracenylidene (AN) chromophores into linear polystyrene chains.
Purity:
>97% (NMR)
Signal word:
Danger
SMILES:
ClCC1=C2C(C=CC=C2)=C(CCl)C3=CC=CC=C31
Solubility Chemicals:
Soluble in DMSO or hot toluene.
Source / Host:
Synthetic
Transportation:
Excepted Quantity
UN Nummer:
1759
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at RT.
References
(1) E.J. Gabe; Acta Cryst. B27, 1925 (1971) | (2) M.G.Krakovyak, et al.; Polymer Science U.S.S.R. 26, 2315 (1984) | (3) E.S. Tillman, et al.; J. Polymer Chem. A 39, 3121 (2001) | (4) N.A. Kuznetsova, et al.; RUss. J. Gen. Chem. 71, 36 (2001)| (5) S.S. Rane, et al.; Pharm. Res. 25, 1158 (2008) | (6) P. Singh, et al.; J. Fluorescence 24, 417 (2014) | (7) A. Ferrer-Ugalde, et al.; Chemistry 20, 9940 (2014)