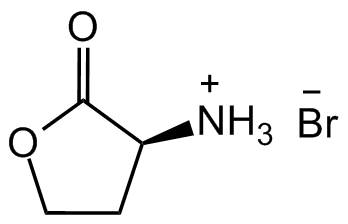
L-Homoserine lactone hydrobromide
| Code | Size | Price |
|---|
| CDX-H0310-G005 | 5 g | £126.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
Short Term: RT Long Term: +4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
(S)-(-)-alpha-Amino-gamma-butyrolactone hydrobromide; L-Homoserine lactone HBr
Appearance:
White to beige powder.
CAS:
15295-77-9
EClass:
32160000
Form (Short):
solid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C4H7NO2.BrH/c5-3-1-2-7-4(3)6;/h3H,1-2,5H2;1H/t3-;/m0./s1
InChiKey:
MKLNTBLOABOJFZ-DFWYDOINSA-N
Long Description:
Chemical. CAS: 15295-77-9. Formula: C4H7NO2 . HBr. MW: 182.02. L-Homoserine lactone hydrobromide is used as a building block. It can be used to prepare beta-ketoamide N-acylated-L-homoserine lactones (AHLs) as quorum sensing molecules, p-coumaroyl-HSL (homoserine lactone), calpain and lipid peroxidation inhibitors and pseudopeptide inhibitors for Ras farnesyl-protein transferase, as well as selenomethionine.
MDL:
MFCD00674493
Molecular Formula:
C4H7NO2 . HBr
Molecular Weight:
182.02
Package Type:
Vial
Product Description:
L-Homoserine lactone hydrobromide is used as a building block. It can be used to prepare beta-ketoamide N-acylated-L-homoserine lactones (AHLs) as quorum sensing molecules, p-coumaroyl-HSL (homoserine lactone), calpain and lipid peroxidation inhibitors and pseudopeptide inhibitors for Ras farnesyl-protein transferase, as well as selenomethionine.
Purity:
>95% (NMR)
SMILES:
O=C1[C@@H]([NH3+])CCO1.[Br-]
Solubility Chemicals:
Soluble in water (20mg/ml).
Source / Host:
Synthetic
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
(1) T. Koch & O. Buchardt; Synthesis 1065-1067 (1993) | (2) S.L Graham, et al.; J. Med. Chem. 37,725 (1994) | (3) S. Auvin, et al.; Bioorg. Med. Chem. Lett. 14, 3825 (2004) | (4) A.L. Schaefer, et al.; Nature 454, 595 (2008) | (5) J.T. Hodgkinson, et al. Tetrahedr. Lett. 52, 3291 (2011) | (6) D. Jakubczyk, et. al.; Eur. J. Org. Chem. 592, 2014 (2014) | (7) N. Nandakumar, et al.; Bioorg. Med. Chem. Lett. 27, 2967 (2017) | (8) G. Wu, et al.; Chem. Commun. 55, 7860 (2019) | (9) L. Ziesche, et al.; Mar. Drugs 17, 20 (2019)