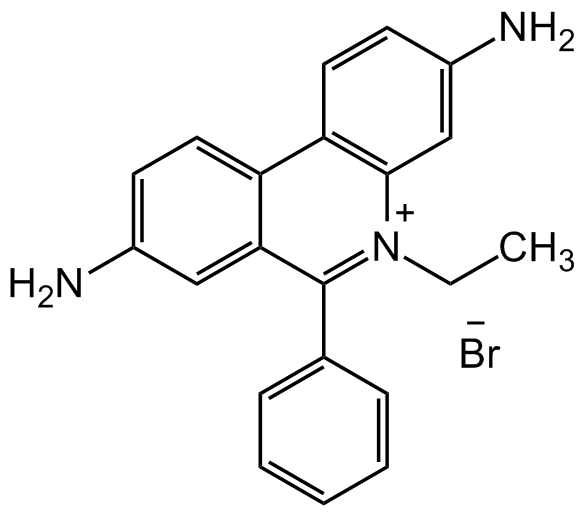
Ethidium bromide
| Code | Size | Price |
|---|
| CDX-E0005-G001 | 1 g | £57.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
Short Term: RT, Long Term: +4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
3,8-Diamino-5-ethyl-6-phenylphenanthridinium bromide; EtBr; Homidium bromide
Appearance:
Dark violet powder.
CAS:
1239-45-8
Class:
6.1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS06,GHS08
Handling Advice:
Protect from light and moisture.
Hazards:
H302-H330-H341
InChi:
InChI=1S/C21H19N3.BrH/c1-2-24-20-13-16(23)9-11-18(20)17-10-8-15(22)12-19(17)21(24)14-6-4-3-5-7-14;/h3-13,23H,2,22H2,1H3;1H
InChiKey:
ZMMJGEGLRURXTF-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 1239-45-8. Formula: C21H20BrN3. MW: 394.31. Ethidium bromide (EtBr) is the most commonly used nucleic acid stain for PAGE or agarose gel electrophoresis. The fluorescence of EtBr increases 21-fold upon binding to double-stranded RNA and 25-fold on binding double-stranded DNA so that destaining the background is not necessary with a low stain concentration (10 µg/ml). Ethidium bromide has been used in a number of fluorimetric assays for nucleic acids. Ethidium bromide intercalates double-stranded DNA and RNA and acts as a frameshift mutagen. It confers a deep red stain to DNA by acting as an intercalating agent between the starch bases. It has been shown to bind to single-stranded DNA (although not as strongly) and triple-stranded DNA. Because of its ability to bind to DNA, EtBr is an inhibitor of DNA polymerase. It can be used in conjunction with acridine orange to differentiate between viable, apoptotic and necrotic cells. Has been used in the past as an anti-parasitic and anti-microbial agent and described as an uncoupler of oxidative phosphorylation. Has also been used to stain mitochondrial DNA. For staining a gel after electrophoresis, dilute a sample of the stock solution to 0.5µg/ml with water and incubate the gel for 15-30 min. Destaining is usually not needed but can be carried out in water for 15min if decreased background is necessary. The DNA bands can then be detected on a UV light box (254nm wavelength). Ethidium bromide can also be incorporated into the gel and running buffer at 0.5µg/ml and visualized immediately after electrophoresis. Spectral data: lambdaEx=480nm; lambdaEm=620nm (in H2O). lambdaEx=518nm; lambdaEm=605nm (bound to DNA). lambdaEx=530nm; lambdaEm=600nm (in 50mM phosphate buffer pH 7.0; upon binding to DNA).
MDL:
MFCD00011724
Molecular Formula:
C21H20BrN3
Molecular Weight:
394.31
Package Type:
Vial
PG:
I
Precautions:
P260-P281-P284-P310
Product Description:
Ethidium bromide (EtBr) is the most commonly used nucleic acid stain for PAGE or agarose gel electrophoresis. The fluorescence of EtBr increases 21-fold upon binding to double-stranded RNA and 25-fold on binding double-stranded DNA so that destaining the background is not necessary with a low stain concentration (10 µg/ml). Ethidium bromide has been used in a number of fluorimetric assays for nucleic acids. Ethidium bromide intercalates double-stranded DNA and RNA and acts as a frameshift mutagen. It confers a deep red stain to DNA by acting as an intercalating agent between the starch bases. It has been shown to bind to single-stranded DNA (although not as strongly) and triple-stranded DNA. Because of its ability to bind to DNA, EtBr is an inhibitor of DNA polymerase. It can be used in conjunction with acridine orange to differentiate between viable, apoptotic and necrotic cells. Has been used in the past as an anti-parasitic and anti-microbial agent and described as an uncoupler of oxidative phosphorylation. Has also been used to stain mitochondrial DNA. For staining a gel after electrophoresis, dilute a sample of the stock solution to 0.5µg/ml with water and incubate the gel for 15-30 min. Destaining is usually not needed but can be carried out in water for 15min if decreased background is necessary. The DNA bands can then be detected on a UV light box (254nm wavelength). Ethidium bromide can also be incorporated into the gel and running buffer at 0.5µg/ml and visualized immediately after electrophoresis. Spectral data: lambdaEx=480nm; lambdaEm=620nm (in H2O). lambdaEx=518nm; lambdaEm=605nm (bound to DNA). lambdaEx=530nm; lambdaEm=600nm (in 50mM phosphate buffer pH 7.0; upon binding to DNA).
Purity:
>95% (HPLC)
Signal word:
Danger
SMILES:
NC(C=C1)=CC2=C1C3=CC=C(N)C=C3C(C4=CC=CC=C4)=[N+]2CC.[Br-]
Solubility Chemicals:
Soluble in water (10mg/ml).
Source / Host:
Synthetic
Transportation:
Excepted
UN Nummer:
2811PIH
UNSPSC Category:
Fluorescent Reagents
UNSPSC Number:
41105331
Use & Stability:
This product should not be used if colour has changed or if there are
References
(1) L.J. Eron & B.R. McAuslan; Biochim. Biophys. Acta 114, 633 (1966) | (2) J.A. Donkersloot, et al.; Appl. Microbiol. 24, 179 (1972) | (3) J.B. Le Pecq; Methods Biochem. Anal. 20, 41 (1971) (Review) | (4) J.C. Stockert; Naturwissenschaften 61, 363 (1974) | (5) M. Miko & B. Chance; FEBS Lett. 54, 347 (1975) | (6) A.R. Morgan, et al.; Nucleic Acids Res. 7, 547 (1979) (Review) | (7) A.R. Morgan, et al.; Nucleic Acids Res. 7, 571 (1979) (Review) | (8) A. Vincent & K. Scherrer; Mol. Biol. Rep. 5, 209 (1979) | (9) W.A. Franklin & J.D. Locker; J. Histochem. Cytochem. 29, 572 (1981) | (10) S. de Jong, et al.; Int. J. Cancer 37, 557 (1986) | (11) D.W. Gray & P.J. Morris; Stain Technol. 62, 373 (1987) | (12) E.A. Ribeiro, et al.; Anal. Biochem. 181, 197 (1989) | (13) P. Borst; IUBMB Life 57; 745 (2005) (Review) | (14) A.M. Villa & S.M. Doglia; Eur. J. Cancer. 45, 2588 (2009) | (15) A.M. Villa, et al.; J. Biomed. Opt. 17, 046001 (2012)