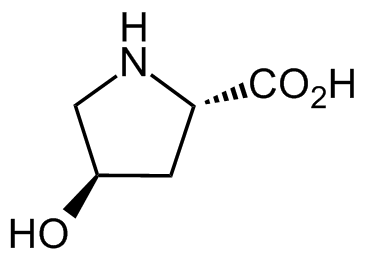
trans-4-Hydroxy-L-proline
| Code | Size | Price |
|---|
| CDX-T0254-G025 | 25 g | £59.00 |
Quantity:
| CDX-T0254-G100 | 100 g | £157.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
+20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
(2S,4R)-4-Hydroxypyrrolidine-2-carboxylic acid; Hyp; (S)-(-)-trans-4-Hydroxyproline; 4(R)-Hydroxy-2(S)-pyrrolidinecarboxylic acid; NSC 46704; H-Hyp-OH; L-Hydroxypyroline
Appearance:
White to off-white powder.
CAS:
51-35-4
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C5H9NO3/c7-3-1-4(5(8)9)6-2-3/h3-4,6-7H,1-2H2,(H,8,9)/t3-,4?/m1/s1
InChiKey:
PMMYEEVYMWASQN-SYPWQXSBSA-N
Long Description:
Chemical. CAS: 51-35-4. Formula: C5H9NO3. MW: 131.13. Trans-4-Hydroxy-L-proline is a non-essential amino acid and a natural constituent of animal structural proteins such as collagen and elastin. It is commonly used as a chiral building block in the organic synthesis of various compounds, such as neuroexcitatory kainoids and antifungal echinocandins. It has been found in many bacterial secondary metabolites, including echinocandins.
MDL:
MFCD00064320
Molecular Formula:
C5H9NO3
Molecular Weight:
131.13
Package Type:
Vial
Product Description:
Trans-4-Hydroxy-L-proline is a non-essential amino acid and a natural constituent of animal structural proteins such as collagen and elastin. It is commonly used as a chiral building block in the organic synthesis of various compounds, such as neuroexcitatory kainoids and antifungal echinocandins. It has been found in many bacterial secondary metabolites, including echinocandins.
Purity:
>99% (TLC)
SMILES:
O[C@H]1CN[C@H](C(O)=O)C1
Solubility Chemicals:
Soluble in water.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at RT.
References
(1) P. Remuzon, et al.; Tetrahedron 44, 13803 (1996) | (2) M.J. Blanco & F.J. Sardina; J. Org. Chem. 61, 4748 (1996) | (3) J.E. Baldwin, et al.; Bioorg. Med. Chem. Lett. 10, 1927 (2000) | (4) J.F. Poisson, et al.; J. Org. Chem. 70, 10860 (2005) | (5) G. Cremonesi, et al.; J. Org. Chem. 75, 2010 (2010)