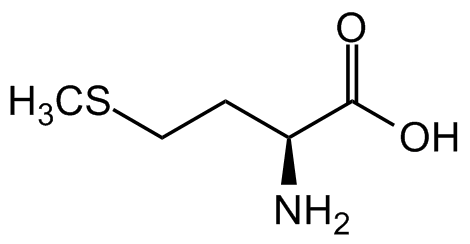
L-Methionine
| Code | Size | Price |
|---|
| CDX-M0524-G025 | 25 g | £35.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
+20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
(S)-2-Amino-4-(methylmercapto)butyric acid; L-2-Amino-4-(methylthio)butanoic acid; S-methyl-L-Homocysteine; NSC 22946; h-Met-oh
Appearance:
White powder.
CAS:
63-68-3
EClass:
32160000
Form (Short):
solid
Handling Advice:
Protect from light and moisture.
Hazards:
H315-H319-H335
InChi:
InChI=1S/C5H11NO2S/c1-9-3-2-4(6)5(7)8/h4H,2-3,6H2,1H3,(H,7,8)/t4-/m0/s1
InChiKey:
FFEARJCKVFRZRR-BYPYZUCNSA-N
Long Description:
Chemical. CAS: 63-68-3. Formula: C5H11NO2S. MW: 149.21. Essential amino acid involved in various biochemical processes in humans and mammals. Important in angiogenesis, the growth of new blood vessels and supplementation may be beneficial in diseases such as Parkinson's, schizophrenia, asthma or allergies, as well as in radiation, copper poisoning, alcoholism or depressions. Through transmethylation it can be converted to S-adenosylmethionine, a primary methyl donor that methylates compounds to form such products as creatine and phosphatidylcholine. Methionine can be converted to cysteine, a precursor of glutathione and taurine. These molecules may regulate intestinal epithelial oxidative status, and may contribute to intestinal mucosal integrity and gut function. Overconsumption of methionine has been associated to cancer growth. Restriction of methionine can increase lifespans and inhibit cancer growth. Used in livestock nutrition. Apart from its nutritional function, also important for the metabolism and gut health of animals. L-Methionine serves as precursor for transmethylation and transsulphuration. Methionine adenosylation results in the formation of S-adenosyl-L-methionine (SAM). SAM serves as a methyl donor to a number of substances. This methylation is significantly associated with the immune system functioning. Thus, SAM deficiency causes severe combined immunodeficiencies. L-Methionine?s metabolic product, glutathione, is known to regulate immune response and has antiviral action.
MDL:
MFCD00063097
Molecular Formula:
C5H11NO2S
Molecular Weight:
149.21
Package Type:
Vial
Product Description:
Essential amino acid involved in various biochemical processes in humans and mammals. Important in angiogenesis, the growth of new blood vessels and supplementation may be beneficial in diseases such as Parkinson's, schizophrenia, asthma or allergies, as well as in radiation, copper poisoning, alcoholism or depressions. Through transmethylation it can be converted to S-adenosylmethionine, a primary methyl donor that methylates compounds to form such products as creatine and phosphatidylcholine. Methionine can be converted to cysteine, a precursor of glutathione and taurine. These molecules may regulate intestinal epithelial oxidative status, and may contribute to intestinal mucosal integrity and gut function. Overconsumption of methionine has been associated to cancer growth. Restriction of methionine can increase lifespans and inhibit cancer growth. Used in livestock nutrition. Apart from its nutritional function, also important for the metabolism and gut health of animals. L-Methionine serves as precursor for transmethylation and transsulphuration. Methionine adenosylation results in the formation of S-adenosyl-L-methionine (SAM). SAM serves as a methyl donor to a number of substances. This methylation is significantly associated with the immune system functioning. Thus, SAM deficiency causes severe combined immunodeficiencies. L-Methionine?s metabolic product, glutathione, is known to regulate immune response and has antiviral action.
Purity:
>98% (HPLC)
SMILES:
N[C@H](C(O)=O)CCSC
Solubility Chemicals:
Soluble in water (25mg/ml).
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at RT.
References
(1) P.J. Garlick; J. Nutr. 136, 1722S (2006) | (2) R. Van Brummelen & D. du Toit; Amino Acids 33, 157 (2007) | (3) J. Gomez, et al.; J. Bioenerg. Biomembr. 41, 309 (2009) | (4) A. Karau & I. Grayson; Adv. Biochem. Eng. Biotechnol. 143, 189 (2014)