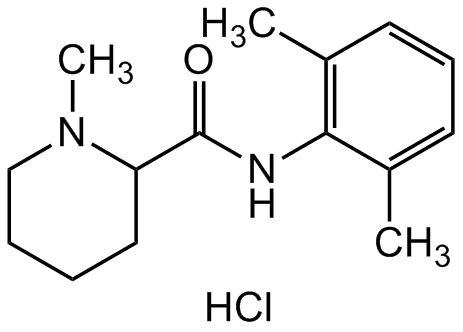
Mepivacaine hydrochloride
| Code | Size | Price |
|---|
| CDX-M0557-M050 | 50 mg | £84.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
+20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
1-Methyl-2',6'-pipecoloxylidine hydrochloride; N-(2,6-Dimethylphenyl)-1-methyl-2-piperidinecarboxamide hydrochloride; (?)-Mepivacaine hydrochloride
Appearance:
White to beige powder.
CAS:
1722-62-9
Class:
6.1
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS06
Handling Advice:
Protect from light and moisture.
Hazards:
H301
InChi:
InChI=1S/C15H22N2O.ClH/c1-11-7-6-8-12(2)14(11)16-15(18)13-9-4-5-10-17(13)3;/h6-8,13H,4-5,9-10H2,1-3H3,(H,16,18);1H
InChiKey:
RETIMRUQNCDCQB-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 1722-62-9. Formula: C15H22N2O . HCl. MW: 282.81. Mepivacaine hydrochloride is a long-acting local anesthetic that reversibly blocks transient Na+ inward current, as well as the steady-state K+ outward current. Mepivacaine blocks tandem pore (TASK) and Kv1.5 potassium channels in model systems.
MDL:
MFCD00144738
Molecular Formula:
C15H22N2O . HCl
Molecular Weight:
282.81
Package Type:
Vial
PG:
III
Precautions:
P301 + P310
Product Description:
Mepivacaine hydrochloride is a long-acting local anesthetic that reversibly blocks transient Na+ inward current, as well as the steady-state K+ outward current. Mepivacaine blocks tandem pore (TASK) and Kv1.5 potassium channels in model systems.
Purity:
>98% (HPLC)
Signal Word:
Danger
SMILES:
CC1=CC=CC(C)=C1NC(C2N(C)CCCC2)=O.Cl
Solubility Chemicals:
Soluble in water (20mg/ml).
Transportation:
Excepted Quantity
UN Nummer:
2811
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at RT.
References
(1) F.P. Luduena, et al.; Toxicol. Appl. Pharmacol. 2, 295 (1960) | (2) M. Sadove & G.D. Wessinger; J. Int. Coll. Surg. 34, 573 (1960) | (3) A. El-Maghraby, et al.; J. Egypt Med. Assoc. 47, 153 (1964) | (4) A.G. Burm, et al.; Anesth. Analg. 84, 85 (1997) | (5) V. Tagariello, et al.; Minerva Anestesiol. 67, 5 (2001) (Review) | (6) A. Leffler, et al.; Eur. J. Pharmacol. 630, 19 (2010) | (7) W.G. Brockmann; Gen. Dent. 62, 70 (2014) (Review)