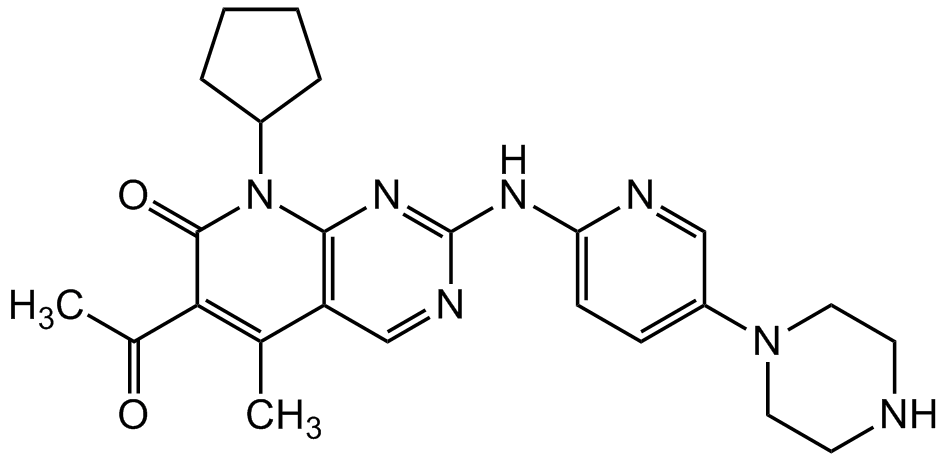
Palbociclib
| Code | Size | Price |
|---|
| CDX-P0590-M005 | 5 mg | £65.00 |
Quantity:
| CDX-P0590-M025 | 25 mg | £237.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
+20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
6-Acetyl-8-cyclopentyl-5-methyl-2-[[5-(1-piperazinyl)-2-pyridinyl]amino]pyrido[2,3-d]pyrimidin-7(8H)-one; PD 0332991; PD-332991; PD991; PF 00080665
Appearance:
Light-yellow or off-yellow powder, beige.
CAS:
571190-30-2
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C24H29N7O2/c1-15-19-14-27-24(28-20-8-7-18(13-26-20)30-11-9-25-10-12-30)29-22(19)31(17-5-3-4-6-17)23(33)21(15)16(2)32/h7-8,13-14,17,25H,3-6,9-12H2,1-2H3,(H,26,27,28,29)
InChiKey:
AHJRHEGDXFFMBM-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 571190-30-2. Formula: C24H29N7O2. MW: 447.53. Palbociclib is a potent and selective inhibitor of CDK4 (IC50=11nM) and CDK6 (IC50=16nM). Displays selectivity for Cdk4/6 over a panel of 36 additional protein kinases. It exhibits selective antiproliferation activity against Rb-positive human breast/colon/lung/leukemia cancer cultures and displays in vivo efficacy against various advanced stage human tumor xenografts in mice. It inhibits phosphorylation of Rb protein and cell cycle progression through G1 in primary 5T33MM cells and sensitized these cells to killing by a proteasome inhibitor (bortezomib) in mouse models. Induces autophagy and senescence in AGS gastric cancer cells. It is a clinically useful breast cancer agent. In addition cell cycle inhibitors have been shown to boost tumor immunogenicity.
MDL:
MFCD11840850
Molecular Formula:
C24H29N7O2
Molecular Weight:
447.53
Package Type:
Vial
Product Description:
Palbociclib is a potent and selective inhibitor of CDK4 (IC50=11nM) and CDK6 (IC50=16nM). Displays selectivity for Cdk4/6 over a panel of 36 additional protein kinases. It exhibits selective antiproliferation activity against Rb-positive human breast/colon/lung/leukemia cancer cultures and displays in vivo efficacy against various advanced stage human tumor xenografts in mice. It inhibits phosphorylation of Rb protein and cell cycle progression through G1 in primary 5T33MM cells and sensitized these cells to killing by a proteasome inhibitor (bortezomib) in mouse models. Induces autophagy and senescence in AGS gastric cancer cells. It is a clinically useful breast cancer agent. In addition cell cycle inhibitors have been shown to boost tumor immunogenicity.
Purity:
>98% (HPLC)
SMILES:
O=C1C(C(C)=O)=C(C)C(C=NC(NC2=CC=C(N3CCNCC3)C=N2)=N4)=C4N1C5CCCC5
Solubility Chemicals:
Soluble in DMSO (2mg/ml).
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at RT.
References
(1) D.W. Fry, et al.; Mol. Cancer Ther. 3, 1427 (2004) | (2) E. Menu, et al.; Cancer Res. 68, 5519 (2008) | (3) R.L. Sutherland & E.A. Musgrove; Breast Cancer Res. 11, 112 (2009) | (4) R.S. Finn, et al.; Breast Cancer Res. 11, R77 (2009) | (5) A. Rocca, et al.; Expert Opin. Pharmacother. 15, 407 (2014) (Review) | (6) J.D. Altenburg & S.S. Farag; Expert Opin. Investig. Drugs 24, 261 (2015) (Review) | (7) A.S. Clark, et al.; JAMA Oncol. 2, 253 (2016) (Review) | (8) C.A. Valenzuela, et al.; Exp. Cell Res. 360, 390 (2017) | (9) S. Goel, et al.; Nature 548, 471 (2017) | (10) M. Schmidt & M. Sebastian; Recent Results Cancer Res. 211, 153 (2018) (Review) | (11) M. Poratti & G. Marzaro; Eur. J. Med. Chem. 172, 143 (2019) (Review)