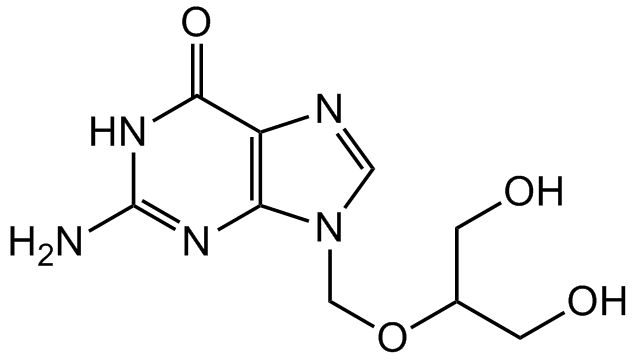
Ganciclovir
| Code | Size | Price |
|---|
| CDX-G0046-M100 | 100 mg | £121.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
2-Amino-1,9-dihydro-9-[[2-hydroxy-1-(hydroxymethyl)ethoxy]methyl]-6H-purin-6-one; 9-(1,3-Dihydroxy-2-propoxymethyl)guanine; DHPG; BW 759; 2'-Nor-2'-deoxyguanosine
Appearance:
White to light yellow powder.
CAS:
82410-32-0
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS08
Handling Advice:
Protect from light and moisture.
Hazards:
H340-H361fd
InChi:
InChI=1S/C9H13N5O4/c10-9-12-7-6(8(17)13-9)11-3-14(7)4-18-5(1-15)2-16/h3,5,15-16H,1-2,4H2,(H3,10,12,13,17)
InChiKey:
IRSCQMHQWWYFCW-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 82410-32-0. Formula: C9H13N5O4. MW: 255.23. Ganciclovir is antiviral synthetic analog of the nucleoside 2'-deoxy-guanosine, which is used to treat or prevent cytomegalovirus (CMV) infections. It acts as a prodrug activated by phosphorylation. It is first phosphorylated to ganciclovir monophosphate by a viral kinase encoded by the cytomegalovirus (CMV) gene UL97 during infection. Subsequently, cellular kinases catalyze the formation of ganciclovir diphosphate and ganciclovir triphosphate. Ganciclovir triphosphate is a competitive inhibitor of deoxyguanosine triphosphate (dGTP) incorporation into DNA and preferentially inhibits viral DNA polymerases more than cellular DNA polymerases. It is also useful in the study of gene therapy in cancer research. Ganciclovir has been used in the study of "suicide" gene therapy in cancer research, where upon expression of a viral suicide gene encoding herpes simplex virus, thymidine kinase (TK), the non-toxic prodrug ganciclovir, is converted to an active phosphorylated analog that can be incorporated into the DNA of replicating eukaryotic cells, causing death of the malignant dividing cell. Causes an irreversible cell cycle arrest at the G2/M checkpoint. Ganciclovir has also been used to study the loss of telomeres and to evaluate sensitivity of viruses to antiviral agents.
MDL:
MFCD00870588
Molecular Formula:
C9H13N5O4
Molecular Weight:
255.23
Package Type:
Vial
Precautions:
P201-P202-P280-P308 + P313-P405-P501
Product Description:
Ganciclovir is antiviral synthetic analog of the nucleoside 2'-deoxy-guanosine, which is used to treat or prevent cytomegalovirus (CMV) infections. It acts as a prodrug activated by phosphorylation. It is first phosphorylated to ganciclovir monophosphate by a viral kinase encoded by the cytomegalovirus (CMV) gene UL97 during infection. Subsequently, cellular kinases catalyze the formation of ganciclovir diphosphate and ganciclovir triphosphate. Ganciclovir triphosphate is a competitive inhibitor of deoxyguanosine triphosphate (dGTP) incorporation into DNA and preferentially inhibits viral DNA polymerases more than cellular DNA polymerases. It is also useful in the study of gene therapy in cancer research. Ganciclovir has been used in the study of "suicide" gene therapy in cancer research, where upon expression of a viral suicide gene encoding herpes simplex virus, thymidine kinase (TK), the non-toxic prodrug ganciclovir, is converted to an active phosphorylated analog that can be incorporated into the DNA of replicating eukaryotic cells, causing death of the malignant dividing cell. Causes an irreversible cell cycle arrest at the G2/M checkpoint. Ganciclovir has also been used to study the loss of telomeres and to evaluate sensitivity of viruses to antiviral agents.
Purity:
>99% (HPLC)
Signal Word:
Danger
SMILES:
O=C1C2=C(N(COC(CO)CO)C=N2)N=C(N)N1
Solubility Chemicals:
Soluble in 0.1 N HCl (10mg/ml). Slightly soluble in DMSO (1mg/ml).
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
(1) T. Matthews & R. Boehme; Rev. Infect. Dis. 10, S490 (1988) (Review) | (2) S. Paul & S. Dummer; Am. J. Med. Sci. 304, 272 (1992) (Review) | (3) P.J. Halloran & R.G. Fenton; Cancer Res. 58, 3855 (1998) | (4) J. Qiao, et al.; Hum. Gene Ther. 11, 1569 (2000) | (5) M. Aghi, et al.; J. Gene Med. 2, 148 (2000) (Review) | (6) C. Fillat, et al.; Curr. Gene Ther. 3, 13 (2003) (Review)