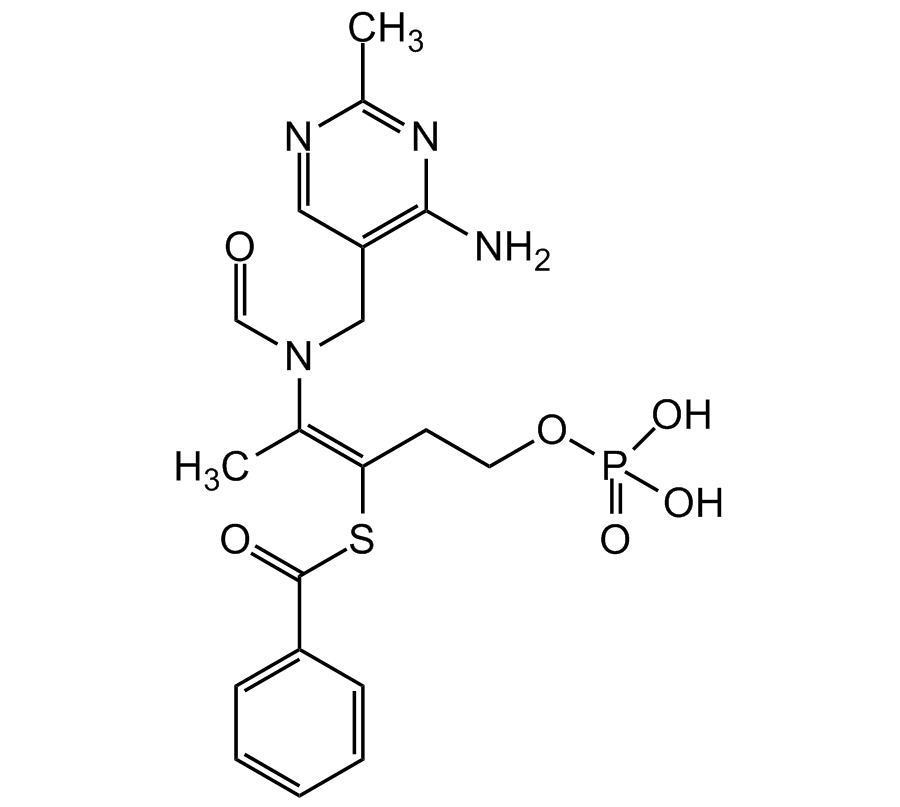
S-Benzoylthiamine O-monophosphate
| Code | Size | Price |
|---|
| CDX-B0403-G001 | 1 g | £84.00 |
Quantity:
| CDX-B0403-G005 | 5 g | £280.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Benfotiamine; Benzoylthiamine monophosphate
Appearance:
White to off-white powder.
CAS:
22457-89-2
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Protect from light and moisture.
Hazards:
H351
InChi:
InChI=1S/C19H23N4O6PS/c1-13(23(12-24)11-16-10-21-14(2)22-18(16)20)17(8-9-29-30(26,27)28)31-19(25)15-6-4-3-5-7-15/h3-7,10,12H,8-9,11H2,1-2H3,(H2,20,21,22)(H2,26,27,28)/b17-13+
InChiKey:
BTNNPSLJPBRMLZ-GHRIWEEISA-N
Long Description:
Chemical. CAS: 22457-89-2. Formula: C19H23N4O6PS. MW: 466.45. S-Benzoylthiamine O-monophosphate is a lipid-soluble form of vitamin B1 (thiamine). S-Benzoylthiamine O-monophosphate facilitates the action of thiamine diphosphate, a cofactor for the enzyme transketolase. The activation of transketolase enzyme accelerates the precursors of advanced glycation end products (AGEs) towards the pentose phosphate pathway thereby reducing the production of AGEs. The reduction in AGEs subsequently decreases metabolic stress which benefits vascular complications seen in diabetes. The anti-AGE property of S-Benzoylthiamine O-monophosphate certainly makes it effective for the treatment of diabetic neuropathy, nephropathy and retinopathy. S-Benzoylthiamine O-monophosphate appears to have a also cardioprotective role in potentiating angiogenesis and inhibiting apoptosis in diabetic complications. S-Benzoylthiamine O-monophosphate also modulates pathways such as arachidonic acid (AA), nuclear transcription Factor kappaB (NF-kappaB), protein kinase B, mitogen-activated protein kinases (MAPK) and vascular endothelial growth factor receptor 2 (VEGFR2) signaling pathways, which are also responsible for it's anti-inflammatory and anti-tumor properties. S-Benzoylthiamine O-monophosphate has been shown to be a dual inhibitory action on COX-2 and LOX-5, supressing PKC and NF-kappaB activation, preventing the downregulation of PKB/Akt during diabetic complications and enhancing cell survival, regulating GSK-3 activity through Akt (rendering it's anti-AD activity) and inducing cell death through paraptosis.
MDL:
MFCD00057343
Molecular Formula:
C19H23N4O6PS
Molecular Weight:
466.45
Package Type:
Vial
Product Description:
S-Benzoylthiamine O-monophosphate is a lipid-soluble form of vitamin B1 (thiamine). S-Benzoylthiamine O-monophosphate facilitates the action of thiamine diphosphate, a cofactor for the enzyme transketolase. The activation of transketolase enzyme accelerates the precursors of advanced glycation end products (AGEs) towards the pentose phosphate pathway thereby reducing the production of AGEs. The reduction in AGEs subsequently decreases metabolic stress which benefits vascular complications seen in diabetes. The anti-AGE property of S-Benzoylthiamine O-monophosphate certainly makes it effective for the treatment of diabetic neuropathy, nephropathy and retinopathy. S-Benzoylthiamine O-monophosphate appears to have a also cardioprotective role in potentiating angiogenesis and inhibiting apoptosis in diabetic complications. S-Benzoylthiamine O-monophosphate also modulates pathways such as arachidonic acid (AA), nuclear transcription Factor kappaB (NF-kappaB), protein kinase B, mitogen-activated protein kinases (MAPK) and vascular endothelial growth factor receptor 2 (VEGFR2) signaling pathways, which are also responsible for it's anti-inflammatory and anti-tumor properties. S-Benzoylthiamine O-monophosphate has been shown to be a dual inhibitory action on COX-2 and LOX-5, supressing PKC and NF-kappaB activation, preventing the downregulation of PKB/Akt during diabetic complications and enhancing cell survival, regulating GSK-3 activity through Akt (rendering it's anti-AD activity) and inducing cell death through paraptosis.
Purity:
>98% (HPLC)
SMILES:
CC1=NC(N)=C(CN(/C(C)=C(CCOP(O)(O)=O)/SC(C2=CC=CC=C2)=O)C=O)C=N1
Solubility Chemicals:
Slightly soluble in DMSO (1mg/ml).
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
(1) E. Beltramo, et al.; Acta Diabetol. 45, 131 (2008) | (2) P. Balakumar, et al.; Pharmacol. Res. 61, 482 (2010) | (3) V. Raj, et al.; Eur. Rev. Med. Pharmacol. Sci. 22, 3261 (2018) (Review)