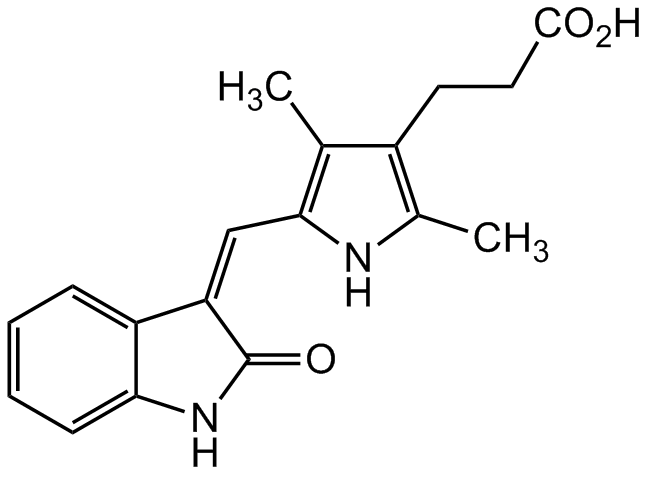
Orantinib
| Code | Size | Price |
|---|
| CDX-O0202-M025 | 25 mg | £280.00 |
Quantity:
| CDX-O0202-M100 | 100 mg | £817.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
TSU68; SU 6668; NSC 702827; 5-[1,2-Dihydro-2-oxo-3H-indol-3-ylidene)methyl]-2,4-dimethyl-1H-pyrrole-3-propanoic acid
Appearance:
Orange to red solid.
CAS:
252916-29-3
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C18H18N2O3/c1-10-12(7-8-17(21)22)11(2)19-16(10)9-14-13-5-3-4-6-15(13)20-18(14)23/h3-6,9,19H,7-8H2,1-2H3,(H,20,23)(H,21,22)/b14-9-
InChiKey:
NHFDRBXTEDBWCZ-ZROIWOOFSA-N
Long Description:
Chemical. CAS: 252916-29-3. Formula: C18H18N2O3. MW: 310.35. Orantinib is an orally bioavailable receptor tyrosine kinase inhibitor. Orantinib binds to and inhibits the autophosphorylation of vascular endothelial growth factor receptor 2 (VEGFR2), platelet-derived growth factor receptor (PDGFR), and fibroblast growth factor receptor (FGFR), thereby inhibiting angiogenesis and cell proliferation. It is a potent ATP-competitive inhibitor of PDGFRbeta, VEGFR2, and FGFR1 (IC50 = 0.06, 2.4, and 3.0 µM, respectively) but not EGFR (IC50 >100 µM). Orantinib suppresses tumor growth, blocks angiogenesis in tumors and induces apoptosis of tumor vasculature and regression of established tumors. Orantinib inhibits the phosphorylation of the stem cell factor receptor tyrosine kinase c-kit, often expressed in acute myelogenous leukemia cells, inhibits Aurora kinases B and C (IC50=35 and 210nM, respectively), and inhibits Unc-51-like serine/threonine kinase Ulk3, involved in hedgehog signaling. All together ornatinib exhibits antiangiogenic, anti-inflammatory, antimetastatic and proapoptotic activity.
MDL:
MFCD09743433
Molecular Formula:
C18H18N2O3
Molecular Weight:
310.35
Package Type:
Vial
Product Description:
Orantinib is an orally bioavailable receptor tyrosine kinase inhibitor. Orantinib binds to and inhibits the autophosphorylation of vascular endothelial growth factor receptor 2 (VEGFR2), platelet-derived growth factor receptor (PDGFR), and fibroblast growth factor receptor (FGFR), thereby inhibiting angiogenesis and cell proliferation. It is a potent ATP-competitive inhibitor of PDGFRbeta, VEGFR2, and FGFR1 (IC50 = 0.06, 2.4, and 3.0 µM, respectively) but not EGFR (IC50 >100 µM). Orantinib suppresses tumor growth, blocks angiogenesis in tumors and induces apoptosis of tumor vasculature and regression of established tumors. Orantinib inhibits the phosphorylation of the stem cell factor receptor tyrosine kinase c-kit, often expressed in acute myelogenous leukemia cells, inhibits Aurora kinases B and C (IC50=35 and 210nM, respectively), and inhibits Unc-51-like serine/threonine kinase Ulk3, involved in hedgehog signaling. All together ornatinib exhibits antiangiogenic, anti-inflammatory, antimetastatic and proapoptotic activity.
Purity:
>98% (HPLC)
SMILES:
O=C1NC2=CC=CC=C2/C1=C/C3=C(C)C(CCC(O)=O)=C(C)N3
Solubility Chemicals:
Soluble in DMSO (10mg/ml) or DMF (5mg/ml).
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) L. Sun, et al.; J. Med. Chem. 42, 5120 (1999) | (2) A.D. Laird, et al.; Cancer Res. 60, 4152 (2000) | (3) B.D. Smolich, et al.; Blood 97, 1413 (2001) | (4) A.D. Laird, et al.; FASEB J. 16, 681 (2002) | (5) K. Godl, et al.; Cancer Res. 65, 6919 (2005) | (6) D.W. Kim, et al.; J. Clin. Endocrinol. Metab. 91, 4070 (2006) | (7) J. Bain, et al.; Biochem. J. 408, 297 (2007) | (8) M. Yamamoto, et al.; Cancer Res. 68, 9754 (2008) | (9) N. Kammasud, et al.; Bioorg. Med. Chem. Lett. 19, 745 (2009) | (10) A. Piirsoo, et al.; Biochim. Biophys. Acta 1843, 703 (2014) | (11) L. Kasak, et al.; Biochem. 57, 5456 (2018)