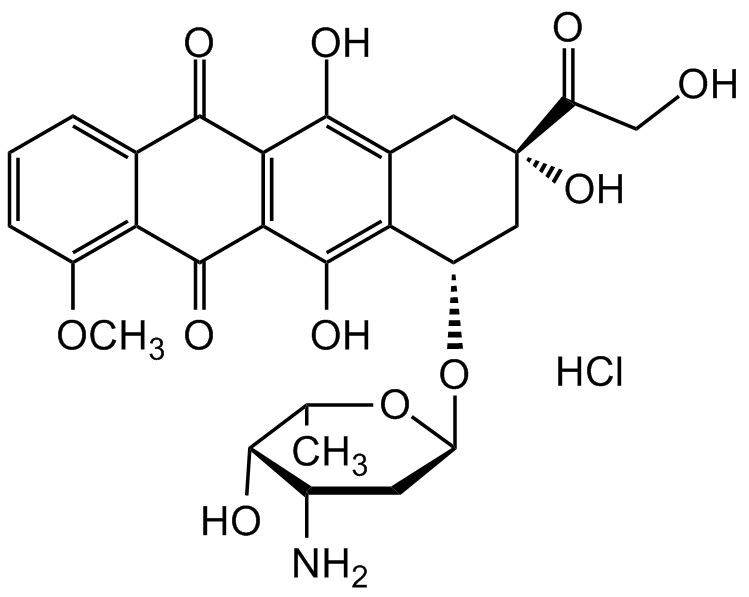
Doxorubicin hydrochloride
| Code | Size | Price |
|---|
| CDX-D0257-M010 | 10 mg | £59.00 |
Quantity:
| CDX-D0257-M050 | 50 mg | £126.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
Short Term: +20°C, Long Term: +4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
DOX; Hydroxydaunorubicin; Adriamycin; NSC 123127
Appearance:
Orange to light red powder.
CAS:
25316-40-9
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07,GHS08
Handling Advice:
Protect from light and moisture.
Hazards:
H225 - H319
InChi:
InChI=1S/C27H29NO11.ClH/c1-10-22(31)13(28)6-17(38-10)39-15-8-27(36,16(30)9-29)7-12-19(15)26(35)21-20(24(12)33)23(32)11-4-3-5-14(37-2)18(11)25(21)34;/h3-5,10,13,15,17,22,29,31,33,35-36H,6-9,28H2,1-2H3;1H/t10-,13-,15-,17-,22+,27-;/m0./s1
InChiKey:
MWWSFMDVAYGXBV-RUELKSSGSA-N
Long Description:
Chemical. CAS: 25316-40-9. Formula: C27H29NO11 . HCl. MW: 579.98. Doxorubicin, a broad spectrum anthracycline antibiotic, inhibits DNA and RNA synthesis in mammalian cells and has been shown to be a very effective anti-tumor agent. Doxorubicin binds to nucleic acids by intercalating the DNA double helix and stabilizing topoisomerase II cleavage complexes, leading to DNA strand breaks at specific doxorubicin-induced sites and formation of reactive oxygen species (ROS) in cells. It also been shown to evict histones leading to chromatin damage. Doxorubicin induces apoptosis by inducing the accumulation of the p53 tumor suppressor protein and has been shown to have immunosuppressive properties. DOX can reduce mitochondrial NADH accumulation and impair oxidative phosphorylation in heart tissues, events associated with reduced glucose uptake. Doxorubicin can also induce the opening of mitochondrial permeability transition pore, resulting in the loss of mitochondrial membrane potential, thus explaining DOX-mediated apoptosis in cardiomyocytes. Doxorubicin is a substrate of MRP1. Doxorubicin shows antimalarial activity and has been shown to inhibit parasite growth. Doxorubicin is used in the treatment of non-Hodgkin?s lymphoma and other cancers. Doxorubicin is naturally fluorescent with lambdaex at 480nm and lambdaem at 600nm. The fluorescent property has been exploited for the measurement of drug efflux pump activities as well as resolving the important question of intracellular localization of various multidrug resistance proteins and the role of subcellular organelles (Golgi and lysosome) in the sequestration of drugs and its implication in drug resistant phenotypes.
MDL:
MFCD00077757
Molecular Formula:
C27H29NO11 . HCl
Molecular Weight:
579.98
Package Type:
Vial
Precautions:
P201-P305 + P351 + P338-P308 + P313
Product Description:
Doxorubicin, a broad spectrum anthracycline antibiotic, inhibits DNA and RNA synthesis in mammalian cells and has been shown to be a very effective anti-tumor agent. Doxorubicin binds to nucleic acids by intercalating the DNA double helix and stabilizing topoisomerase II cleavage complexes, leading to DNA strand breaks at specific doxorubicin-induced sites and formation of reactive oxygen species (ROS) in cells. It also been shown to evict histones leading to chromatin damage. Doxorubicin induces apoptosis by inducing the accumulation of the p53 tumor suppressor protein and has been shown to have immunosuppressive properties. DOX can reduce mitochondrial NADH accumulation and impair oxidative phosphorylation in heart tissues, events associated with reduced glucose uptake. Doxorubicin can also induce the opening of mitochondrial permeability transition pore, resulting in the loss of mitochondrial membrane potential, thus explaining DOX-mediated apoptosis in cardiomyocytes. Doxorubicin is a substrate of MRP1. Doxorubicin shows antimalarial activity and has been shown to inhibit parasite growth. Doxorubicin is used in the treatment of non-Hodgkin?s lymphoma and other cancers. Doxorubicin is naturally fluorescent with lambdaex at 480nm and lambdaem at 600nm. The fluorescent property has been exploited for the measurement of drug efflux pump activities as well as resolving the important question of intracellular localization of various multidrug resistance proteins and the role of subcellular organelles (Golgi and lysosome) in the sequestration of drugs and its implication in drug resistant phenotypes.
Purity:
>98 (HPLC)
Signal word:
Danger
SMILES:
O=C1C2=C(C(O)=C([C@@H](O[C@H]3C[C@H](N)[C@H](O)[C@H](C)O3)C[C@@](C(CO)=O)(O)C4)C4=C2O)C(C5=C(OC)C=CC=C51)=O.Cl
Solubility Chemicals:
Soluble in DMSO (20mg/ml) or water (20mg/ml). Slightly soluble in methanol.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
(1) H.G. Keizer, et al.; Pharmacol. Ther. 47, 219 (1990) (Review) | (2) S. Patel, et al.; Mol. Pharmacol. 52, 658 (1997) | (3) E. Lorenzo, et al.; J. Biol. Chem. 277, 10883 (2002) | (4) S. Wang, et al.; J. Biol. Chem. 279, 25535 (2004) | (5) C. Carvalho, et al.; Curr. Med. Chem. 16, 3267 (2009) (Review) | (6) C.F. Thorn, et al.; Pharmacogenet. Genom. 21, 440 (2011) (Review) | (7) O. Tacar, et al.; J. Pharm. Pharmacol. 65, 157 (2013) (Review) | (8) A.A. Wakharde, et al.; Am. J. Clin. Microbiol. Antimicrob. 1, 1009 (2018) (Review) | (9) X. Qiao, et al.; PNAS 117, 15182 (2020) (Review) | (10) H. Kumari, et al.; Front. Cardiovasc. Med. 7, 56 (2020) (Review)