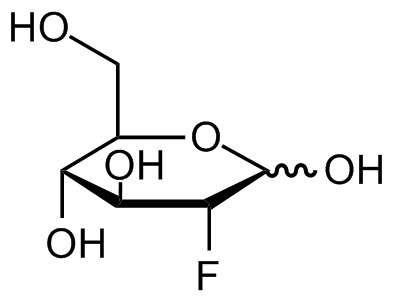
2-Fluoro-2-deoxy-D-glucose
| Code | Size | Price |
|---|
| CDX-F0076-M025 | 25 mg | £114.00 |
Quantity:
| CDX-F0076-M100 | 100 mg | £280.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
Short Term: +4°C, Long Term: -20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
2-Deoxy-2-fluoro-D-glucose; FDG; 2-FG; Fluorodeoxyglucose
Appearance:
White to yellow powder.
CAS:
29702-43-0
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C6H11FO5/c7-3-5(10)4(9)2(1-8)12-6(3)11/h2-6,8-11H,1H2/t2-,3-,4-,5-,6?/m1/s1
InChiKey:
ZCXUVYAZINUVJD-IVMDWMLBSA-N
Long Description:
Chemical. CAS: 29702-43-0. Formula: C6H11FO5. MW: 182.15. 2-Fluoro-2-deoxy-D-glucose [FDG] is a glucose analog that inhibits cellular glycosylation. It is the natural isotope-form of 18F-FDG that can be taken up by cells but does not undergo metabolic glycolysis. FDG uptake is closely related to the expression of the glucose transporter (GLUT) in malignant tumours. FDG is also a substrate of hexokinase isozymes and an inhibitor of cellular glycosylation. FDG is taken up by high-glucose-using cells such as brain, brown adipocytes, kidney and cancer cells, where phosphorylation prevents the glucose from being released again from the cell, once it has been absorbed. The 2-hydroxyl group (?OH) in normal glucose is needed for further glycolysis (metabolism of glucose by splitting it), but FDG is missing this 2-hydroxyl and cannot be further metabolized in cells. FDG is used in positron emission tomography (PET) as contrast agent and is the most widely used PET marker. FDG-PET can be used for diagnosis, staging, and monitoring treatment of cancers, particularly in Hodgkin's disease, non-Hodgkin lymphoma, colorectal cancer, breast cancer, melanoma, and lung cancer. FDG has also anticancer and chemosensitizing activity.
MDL:
MFCD00077527
Molecular Formula:
C6H11FO5
Molecular Weight:
182.15
Package Type:
Vial
Precautions:
P261-P305 + P351 + P338
Product Description:
2-Fluoro-2-deoxy-D-glucose [FDG] is a glucose analog that inhibits cellular glycosylation. It is the natural isotope-form of 18F-FDG that can be taken up by cells but does not undergo metabolic glycolysis. FDG uptake is closely related to the expression of the glucose transporter (GLUT) in malignant tumours. FDG is also a substrate of hexokinase isozymes and an inhibitor of cellular glycosylation. FDG is taken up by high-glucose-using cells such as brain, brown adipocytes, kidney and cancer cells, where phosphorylation prevents the glucose from being released again from the cell, once it has been absorbed. The 2-hydroxyl group (?OH) in normal glucose is needed for further glycolysis (metabolism of glucose by splitting it), but FDG is missing this 2-hydroxyl and cannot be further metabolized in cells. FDG is used in positron emission tomography (PET) as contrast agent and is the most widely used PET marker. FDG-PET can be used for diagnosis, staging, and monitoring treatment of cancers, particularly in Hodgkin's disease, non-Hodgkin lymphoma, colorectal cancer, breast cancer, melanoma, and lung cancer. FDG has also anticancer and chemosensitizing activity.
Purity:
>97% (NMR)
SMILES:
O[C@H]1C(F)C(O)[C@H](O)[C@@H](CO)O1
Solubility Chemicals:
Soluble in DMF (30mg/ml), DMSO (30mg/ml) or water (10mg/ml).
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) H. Minn, et al.; Res. Exp. Med. 191, 27 (1991) | (2) Y. Kanazawa, et al.; J. Neurochem. 66, 2113 (1996) | (3) T.J. Lampidis, et al.; Cancer Chemother. Pharmacol. 58, 725 (2006) | (4) Y. Liu, et al.; Prostate Cancer Prostatic Dis. 9, 230 (2006) | (5) Y. Pina, et al.; Invest. Ophthalmol. Vis. Sci. 53, 996 (2012) | (6) A. Fantagare & A. Svatos; Front. Plant Sci. 7, 483 (2016) | (7) S. Niccoli, et al.; PLosOne 12, e0187584 (2017) | (8) N. Hasnain, et al.; J. Pak. Med. Assoc. 70, 2291 (2020)