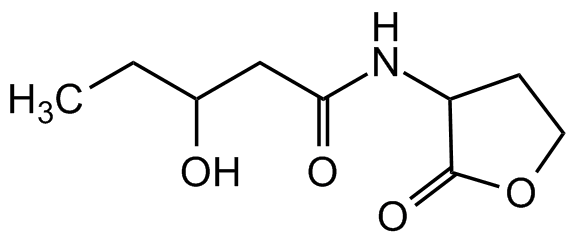
3-Hydroxy-pentanoyl-DL-homoserine lactone
| Code | Size | Price |
|---|
| CDX-H0312-M010 | 10 mg | £72.00 |
Quantity:
| CDX-H0312-M050 | 50 mg | £255.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
Short Term: +4°C, Long Term: -20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
3-OH-C5-HSL; 3-Hydroxy-N-(tetrahydro-2-oxo-3-furanyl)pentanamide
Appearance:
White to off-white solid.
CAS:
148433-24-3
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C9H15NO4/c1-2-6(11)5-8(12)10-7-3-4-14-9(7)13/h6-7,11H,2-5H2,1H3,(H,10,12)
InChiKey:
IZXCXPQWOXJOPE-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 148433-24-3. Formula: C9H15NO4. MW: 201.22. 3-Hydroxy-pentanoyl-DL-homoserine lactone is a small diffusible signaling molecule and is a member of N-acyl-homoserine lactone family. N-acylhomoserine lactones (AHL) are involved in quorum sensing, controlling gene expression, and cellular metabolism. The diverse applications of this kind of molecule include regulation of virulence in general, infection prevention, and formation of biofilms.
MDL:
MFCD24610175
Molecular Formula:
C9H15NO4
Molecular Weight:
201.22
Package Type:
Vial
Product Description:
3-Hydroxy-pentanoyl-DL-homoserine lactone is a small diffusible signaling molecule and is a member of N-acyl-homoserine lactone family. N-acylhomoserine lactones (AHL) are involved in quorum sensing, controlling gene expression, and cellular metabolism. The diverse applications of this kind of molecule include regulation of virulence in general, infection prevention, and formation of biofilms.
Purity:
>97% (GC)
SMILES:
CCC(O)CC(NC1C(OCC1)=O)=O
Solubility Chemicals:
Soluble in chloroform.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) S.R. Chhabra, et al.; J. Antibiot. 46, 441 (1993) | (2) T.R.I. Cataldi, et al.; J. Mass Spec. 43, 82 (2008) | (3) M. Torres, et al.; PLOSone 13, e0195176 (2018) | (4) M.S. Nawaz, et al.; Front. Microbiol. 11, 553621 (2020)