Indocyanine Green [ICG]
| Code | Size | Price |
|---|
| CDX-I0013-M100 | 100 mg | £121.00 |
Quantity:
| CDX-I0013-M500 | 500 mg | £450.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
Short Term: +4°C, Long Term: -20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Cardiogreen; Foxgreen; IC Green; Ujoviridin
Appearance:
Dark-green to black powder.
CAS:
3599-32-4
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C43H48N2O6S2.Na/c1-42(2)38(44(28-14-16-30-52(46,47)48)36-26-24-32-18-10-12-20-34(32)40(36)42)22-8-6-5-7-9-23-39-43(3,4)41-35-21-13-11-19-33(35)25-27-37(41)45(39)29-15-17-31-53(49,50)51;/h5-13,18-27H,14-17,28-31H2,1-4H3,(H-,46,47,48,49,50,51);/q;+1/p-1
InChiKey:
MOFVSTNWEDAEEK-UHFFFAOYSA-M
Long Description:
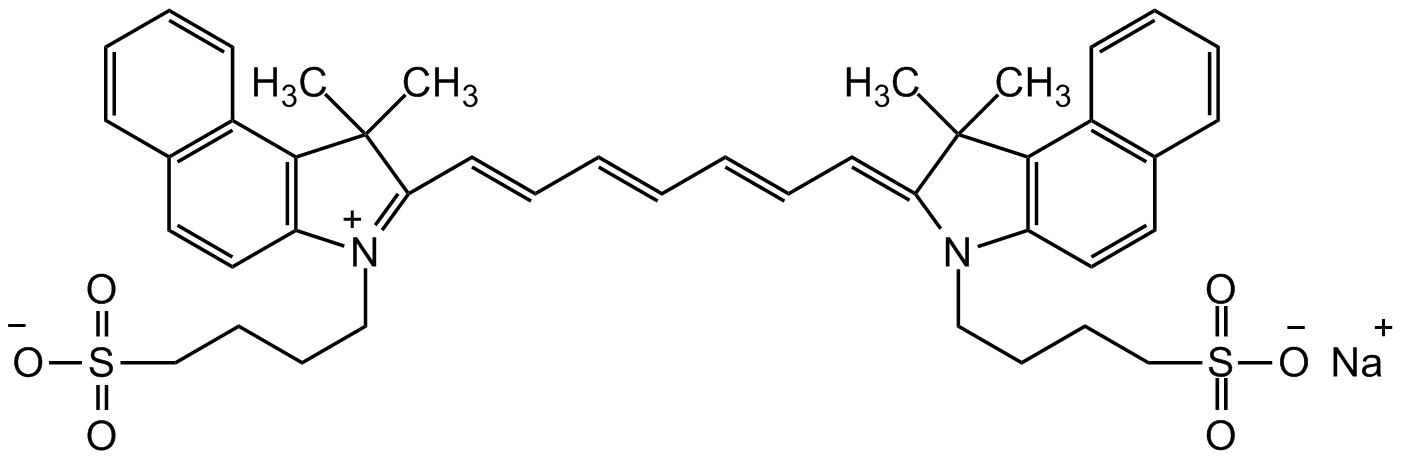
Chemical. CAS: 3599-32-4. Formula: C43H47N2NaO6S2. MW: 774.98. Indocyanine green (ICG) is a cyanine dye. It is a clinically approved near infrared (NIR) fluorescent dye and used in medical diagnostics, in vitro, in vivo and animal model study. NIR fluorescence is being utilized in a wide range of research fields. ICG is a vital fluorescent dye that binds to plasma proteins and lipoproteins. It displays excitation maxima ranging from 750 to 800 nm and an emission maximum of greater than or equal to 800 nm, which shifts from 810-820 nm in aqueous solution to 820-834 nm following intravenous injection. Formulations containing indocyanine green have been commonly used in clinical and surgical applications, including retinal angiography, intraocular surgery, and cardiac output and liver condition monitoring. ICG might be excited using 750-800 nm laser line or LED and displays excellent optical properties. ICG has a half-life of 150 to 180 seconds and is removed from circulation exclusively by the liver. Spectral Data: Ex/Em = 785/812 nm. Extinction coefficient: >220,000 cm-1M-1. CF280: 0.05.
MDL:
MFCD00013078
Molecular Formula:
C43H47N2NaO6S2
Molecular Weight:
774.98
Package Type:
Vial
Precautions:
P261-P305 + P351 + P338
Product Description:
Indocyanine green (ICG) is a cyanine dye. It is a clinically approved near infrared (NIR) fluorescent dye and used in medical diagnostics, in vitro, in vivo and animal model study. NIR fluorescence is being utilized in a wide range of research fields. ICG is a vital fluorescent dye that binds to plasma proteins and lipoproteins. It displays excitation maxima ranging from 750 to 800 nm and an emission maximum of greater than or equal to 800 nm, which shifts from 810-820 nm in aqueous solution to 820-834 nm following intravenous injection. Formulations containing indocyanine green have been commonly used in clinical and surgical applications, including retinal angiography, intraocular surgery, and cardiac output and liver condition monitoring. ICG might be excited using 750-800 nm laser line or LED and displays excellent optical properties. ICG has a half-life of 150 to 180 seconds and is removed from circulation exclusively by the liver. Spectral Data: Ex/Em = 785/812 nm. Extinction coefficient: >220,000 cm-1M-1. CF280: 0.05.
Purity:
>98% (HPLC)
Signal word:
Warning
SMILES:
O=S(CCCCN(C(C=CC1=C2C=CC=C1)=C2C/3(C)C)C3=C/C=C/C=C/C=C/C4=[N+](CCCCS(=O)([O-])=O)C(C=CC5=C6C=CC=C5)=C6C4(C)C)([O-])=O.[Na+]
Solubility Chemicals:
Soluble in DMSO (10mg/ml). Slighlty soluble in ethanol or water (1mg/ml).
Transportation:
Non-hazardous
UNSPSC Category:
Fluorescent Reagents
UNSPSC Number:
41105331
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) T. Desmettre, et al.; Surv. Ophthalmol. 45, 15 (2000) (Review) | (2) M.C. Morales, et al.; Invest. Ophthalmol. Vis. Sci. 51, 6018 (2010) | (3) B.E. Schaafsma, et al.; J. Surg. Oncol. 104, 323 (2011) (Review) | (4) K. Polom, et al.; Cancer 117, 4812 (2011) (Review) | (5) J.T. Alander, et al.; Int. J. Biomed. Imaging 2012, 940585 (2012) (Review) | (6) N. Kokudo & T. Ishizawa; Liver Cancer 1, 15 (2012) (Review) | (7) C. Giraudeau, et al.; Curr. Med. Chem. 21, 1871 (2014) (Review) | (8) J.A. Zelken & A.P. Tufaro; Ann. Surg. Oncol. 22, S1271 (2015) (Review) | (9) M.B. Reinhart, et al.; Surg. Innov. 23, 166 (2016) (Review) | (10) C. Egloff-Juras, et al.; Int. J. Nanomedicine 14, 7823 (2019) (Review) | (11) E. Spartalis, et al.; In Vivo 34, 23 (2020) (Review)