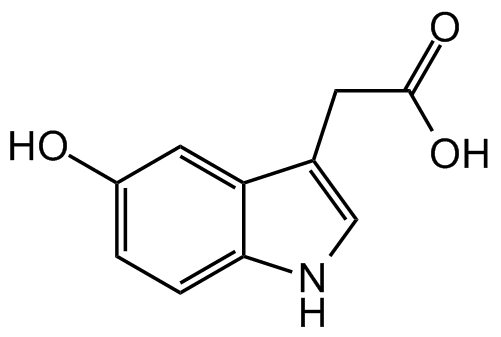
5-Hydroxyindole-3-acetic acid
| Code | Size | Price |
|---|
| AG-CR1-3544-M100 | 100 mg | £45.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
Short Term Storage: +4?C. Long Term Storage: -20?C
Images
Documents
Further Information
Alternate Names/Synonyms:
5-HIAA; 5-Oxyindoleacetic Acid; NSC 90432
Appearance:
Off-white solid to beige/purple solid.
CAS:
54-16-0
EClass:
32160000
Form (Short):
solid
Handling Advice:
Keep cool and dry.
InChi:
InChI=1S/C10H9NO3/c12-7-1-2-9-8(4-7)6(5-11-9)3-10(13)14/h1-2,4-5,11-12H,3H2,(H,13,14)
InChiKey:
DUUGKQCEGZLZNO-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 54-16-0. Formula: C10H9NO3. Molecular Weight: 191.2. 5-hydroxy Indole-3-acetic acid (5-HIAA) is the primary metabolite of serotonin, metabolized by monoamine oxidase and aldehyde dehydrogenase in the liver. As the catabolic end product of the serotonin pathway, 5-HIAA is present in body fluids like cerebrospinal fluid (CSF), blood and urine. It can be used as an internal standard for the quantification of serotonin metabolism. 5-HIAA is a potent GPR35 receptor ligand, an inflammatory mediator for immune and non-immune cell populations. 5-HIAA is secreted by platelets and mast cells during inflammation and by binding to neutrophils has a chemoattractant role. 5-HIAA deficient mice show a loss of GPR35-mediated neutrophil recruitment to inflamed tissue. The chemoattractant GPR35-5-HIAA receptor-ligand system cooperates with other inflammation-induced factors in mediating neutrophil recruitment to sites of inflammation. 5-HIAA is excreted in urine and has been used as a biomarker for the detection of neuroendocrine tumors, Celiac disease and Whipple disease, where serotonin expression levels are increased. It also has been associated with metabolic syndrome and low-grade inflammation. 5-HIAA levels are low in CSF in patients with bipolar 1 disorder with childhood attention-deficit hyperactivity disorder (ADHD) or sepiapterin reductase deficiency. 5-HIAA has also been associated with autism, insomnia and chronic migraine and is used as a marker for alcohol abuse.
MDL:
MFCD00005639
Molecular Formula:
C10H9NO3
Molecular Weight:
191.2
Package Type:
Vial
Product Description:
5-hydroxy Indole-3-acetic acid (5-HIAA) is the primary metabolite of serotonin, metabolized by monoamine oxidase and aldehyde dehydrogenase in the liver. As the catabolic end product of the serotonin pathway, 5-HIAA is present in body fluids like cerebrospinal fluid (CSF), blood and urine. It can be used as an internal standard for the quantification of serotonin metabolism. 5-HIAA is a potent GPR35 receptor ligand, an inflammatory mediator for immune and non-immune cell populations. 5-HIAA is secreted by platelets and mast cells during inflammation and by binding to neutrophils has a chemoattractant role. 5-HIAA deficient mice show a loss of GPR35-mediated neutrophil recruitment to inflamed tissue. The chemoattractant GPR35-5-HIAA receptor-ligand system cooperates with other inflammation-induced factors in mediating neutrophil recruitment to sites of inflammation. 5-HIAA is excreted in urine and has been used as a biomarker for the detection of neuroendocrine tumors, Celiac disease and Whipple disease, where serotonin expression levels are increased. It also has been associated with metabolic syndrome and low-grade inflammation. 5-HIAA levels are low in CSF in patients with bipolar 1 disorder with childhood attention-deficit hyperactivity disorder (ADHD) or sepiapterin reductase deficiency. 5-HIAA has also been associated with autism, insomnia and chronic migraine and is used as a marker for alcohol abuse.
Purity:
>98%
SMILES:
OC1=CC=C(NC=C2CC(O)=O)C2=C1
Solubility Chemicals:
Soluble in DMSO (25mg/ml) or ethanol (25mg/ml).
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Studies on a simplified method for urine 5-hydroxyindole-acetic acid (5-HIAA): C.R. Ratliff; Am. J. Med. Technol. 28, 83 (1962) | CSF serotonin metabolite (5-HIAA) studies in depression, impulsivity, and violence: G.L. Brown & M.I. Linnoila; J. Clin. Psych. 51, 42 (1990) (Review) | Association of biogenic amine metabolites with symptomatology in delusional (psychotic) and nondelusional depressed patients: L. Lykouras, et al.; Prog. Neuropsychopharmacol. Biol. Psych. 19, 877 (1995) | Comparison of self-reported alcohol intake with the urinary excretion of 5-hydroxytryptophol:5-hydroxyindole-3-acetic acid, a biomarker of recent alcohol intake: A. Kroke, et al.; Br. J. Nutr. 85, 621 (2001) | Platelet serotonin levels in pervasive developmental disorders and mental retardation: Diagnostic group differences, within-group distribution, and behavioral correlates: E.J. Mulder, et al.; J. Am. Acad. Child Adolesc. Psychiatry 43, 491 (2004) | Development and validation of an ultra-high performance liquid chromatography-tandem mass-spectrometry (UHPLC-MS/MS) method for the simultaneous determination of neurotransmitters in rat brain samples: R.R. Gonzalez, et al.; J. Neurosci. Meth. 198, 187 (2011) | High plasma 5-hydroxyindole-3-acetic acid concentrations in subjects with metabolic syndrome:M. Fukui, et al.; Diabetes Care 35, 163 (2012) | Association of peripheral 5-hydroxyindole-3-acetic acid, a serotonin derivative, with metabolic syndrome and low-grade inflammation: M. Afarideh, et al.; Endocr. Pract. 21, 711 (2015) | Urinary sampling for 5HIAA and metanephrines determination: revisiting the recommendations: J.-B. Corcuff, et al.; Endocr. Connect. 6, R87 (2017) | Genetic and biochemical changes of the serotonergic system in migraine pathobiology: C.F. Gasparini, et al.; J. Headache Pain 18, 20 (2017) | 5-HIAA as a Potential Biological Marker for Neurological and Psychiatric Disorders: H. Jayamohananan, et al.; Adv. Pharm. Bull. 9, 374 (2019) | GPR35 promotes neutrophil recruitment in response to serotonin metabolite 5-HIAA: M. De Giovanni, et al.; Cell 185, 815 (2022)