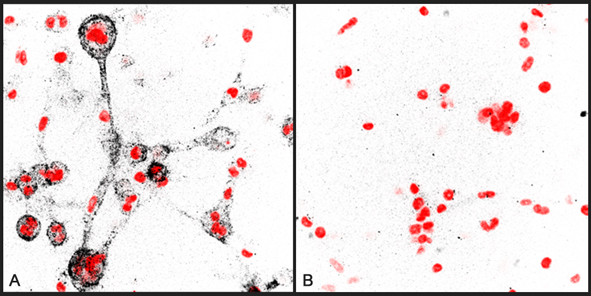
anti-Collagen Type 1 (3/4 fragment) pAb
| Code | Size | Price |
|---|
| AG-25T-0116-C025 | 25 ug | £750.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Host Type: Rabbit
Antibody Clonality: Polyclonal
Regulatory Status: RUO
Target Species:
- Bovine (Cattle)
- Canine (dog)
- Feline (cat)
- Hamster
- Human
- Mouse
- Porcine (pig)
- Rat
- Sheep
Applications:
- Immunocytochemistry (ICC)
- Western Blot (WB)
Shipping:
BLUE ICE
Storage:
Short Term Storage: +4?C. Long Term Storage: -20?C
Images
Documents
Further Information
Alternate Names/Synonyms:
COL1; alpha-1 Type I Collagen
Concentration:
250µg/ml
EClass:
32160000
Form (Short):
liquid
Formulation:
Liquid. In PBS with 1 mg/ml BSA and 0.02% sodium azide.
Handling Advice:
Avoid freeze/thaw cycles.
Immunogen:
Synthetic peptide (human sequence) corresponding to the C-terminal end of the N-terminal three quarter collagen fragment (Col1 ?).
Long Description:
Polyclonal Antibody. Recognizes rat and bovine collagen type 1. Based on sequence identity should also recognize human, mouse, guinea pig, dog, cat, donkey, pig, cow, sheep and chicken collagen type 1. Source: Rabbit. Applications: ICC. Liquid. In PBS with 1 mg/ml BSA and 0.02% sodium azide. The proteolysis of collagens plays an important role in numerous physiological and pathological situations such as morphogenesis, wound healing, arthritis, arteriosclerosis and tumor metastasis. Triple helical type I collagens are made up of two alpha1 (I) and one alpha2 (I) chains, and are found in skin, tendon, ligament and interstitial tissues. Due to their fibrillary structure native collagens are resistant to most proteases. They are substrates for certain matrix metalloproteinases (MMPs), which constitute a family of zinc-dependent enzymes catalyzing the degradation of extracellular matrix components. Initial MMP-8 dependent cleavage of collagen into the characteristic ? and ? fragments has been shown to enable MMP-9 diffusion along the protein helix with preferential binding to the collagen ? fragment tail. Finally, untwisting of the helix end results in the local denaturation of the triple helical structure.
NCBI, Uniprot Number:
N/A
Package Type:
Plastic Vial
Product Description:
The proteolysis of collagens plays an important role in numerous physiological and pathological situations such as morphogenesis, wound healing, arthritis, arteriosclerosis and tumor metastasis. Triple helical type I collagens are made up of two alpha1 (I) and one alpha2 (I) chains, and are found in skin, tendon, ligament and interstitial tissues. Due to their fibrillary structure native collagens are resistant to most proteases. They are substrates for certain matrix metalloproteinases (MMPs), which constitute a family of zinc-dependent enzymes catalyzing the degradation of extracellular matrix components. Initial MMP-8 dependent cleavage of collagen into the characteristic ? and ? fragments has been shown to enable MMP-9 diffusion along the protein helix with preferential binding to the collagen ? fragment tail. Finally, untwisting of the helix end results in the local denaturation of the triple helical structure.
Purity:
Antigen affinity purified.
Source / Host:
Rabbit
Specificity:
Recognizes rat and bovine collagen type 1. Based on sequence identity should also recognize human, mouse, guinea pig, dog, cat, donkey, pig, cow, sheep and chicken collagen type 1.
Transportation:
Non-hazardous
UNSPSC Category:
Primary Antibodies
UNSPSC Number:
12352203
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C.
References
Endosomal WASH and exocyst complexes control exocytosis of MT1-MMP at invadopodia: P. Monteiro, et al.; J. Cell Biol. 203, 1063 (2013) | Physical limits of cell migration: Control by ECM space and nuclear deformation and tuning by proteolysis and traction force: K. Wolf, et al.; J. Cell Biol. 201, 1069 (2013) | Cell jamming: Collective invasion of mesenchymal tumor cells imposed by tissue confinement: A. Haeger, et al.; BBA 1840, 2386 (2014) | Discoidin domain receptor 1 controls linear invadosome formation via a Cdc42?Tuba pathway: A. Juin, et al.; J. Cell Biol. 207, 517 (2014) | Multiparametric Classification Links Tumor Microenvironments with Tumor Cell Phenotype: B. Gligorijevic, et al.; PLoS Biol. 12, e1001995 (2014) | Diverse matrix metalloproteinase functions regulate cancer amoeboid migration: J.L. Orgaz, et al.; Nat. Commun. 5, 4255 (2014) | ARF6?JIP3/4 regulate endosomal tubules for MT1-MMP exocytosis in cancer invasion: V. Marchesin, et al.; J. Cell Biol. 211, 339 (2015) | Flightless I interacts with NMMIIA to promote cell extension formation, which enables collagen remodeling: P.D. Arora, et al.; Mol. Biol. Cell 26, 2279 (2015) | p63/MT1-MMP axis is required for in situ to invasive transition in basal-like breast cancer: C. Lodillinsky, et al. Oncogene 35, 344 (2016) | PI3Kβ links integrin activation and PI(3,4)P2 production during invadopodial maturation: Z. Erami, et al.; Mol. Biol. Cell. 30, 2367 (2019) | Decreased levels of endocytic collagen receptor Endo180 in dermal fibroblasts lead to decreased production of type I collagen and increased expression of matrix metalloproteinase-1: H. Iwahashi, et al.; Photodermatol. Photoimmunol. Photomed. (Epub ahead of print) (2021)