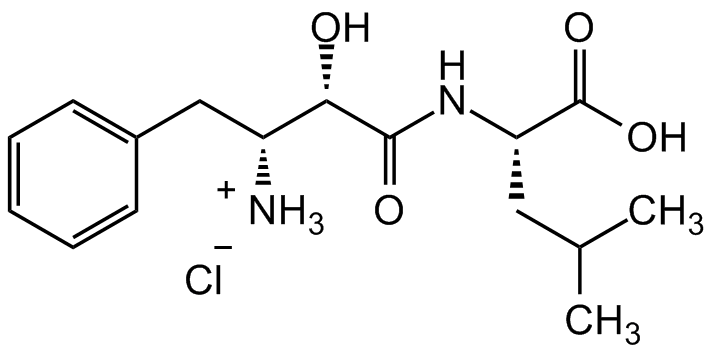
Bestatin . hydrochloride
| Code | Size | Price |
|---|
| AG-CP3-7005-M005 | 5 mg | £55.00 |
Quantity:
| AG-CP3-7005-M025 | 25 mg | £190.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
Short Term Storage: +4?C. Long Term Storage: -20?C
Images
Documents
Further Information
Alternate Names/Synonyms:
Ubenimex; NK421; NSC265489; N-(3R-Amino-2S-hydroxy-1-oxo-4-phenylbutyl)-L-leucine
Appearance:
White solid.
CAS:
65391-42-6
EClass:
32160000
Form (Short):
solid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C16H24N2O4.ClH/c1-10(2)8-13(16(21)22)18-15(20)14(19)12(17)9-11-6-4-3-5-7-11;/h3-7,10,12-14,19H,8-9,17H2,1-2H3,(H,18,20)(H,21,22);1H/t12-,13+,14+;/m1./s1
InChiKey:
XGDFITZJGKUSDK-UDYGKFQRSA-N
Long Description:
Chemical. CAS: 65391-42-6. Formula: C16H24N2O4 . HCl. Molecular Weight: 308.4 . 36.5. Antibiotic. Potent, competitive and specific aminopeptidase inhibitor. Can be administered in vitro to cultured cells or in vivo with low toxicity. Inhibits aminopeptidase B, aminopeptidase N, leucine aminopeptidase and the aminopeptidase activity of leukotriene A4 (LTA4) hydrolase. It is selective for these aminopeptidases over aminopeptidase A, trypsin, chymotrypsin, elastase, papain, pepsin and thermolysin. Immunomodulator. Displays immunostimulant activity via activation of macrophages and T lymphocytes. Displays anticancer and anti-inflammatory activity. Shown to inhibit proliferation, migration and invasion, and induces autophagy and apoptosis in cancer cells. Inhibits the production of LTB4 in erythrocytes. Has been used as useful tool to assess the physiological role of certain mammalian exopeptidases in the regulation of the immune system, the growth of tumors and their invasion of surrounding tissues and the degradation of cellular proteins.
MDL:
MFCD00058004
Molecular Formula:
C16H24N2O4 . HCl
Molecular Weight:
308.4 . 36.5
Package Type:
Vial
Product Description:
Antibiotic. Potent, competitive and specific aminopeptidase inhibitor. Can be administered in vitro to cultured cells or in vivo with low toxicity. Inhibits aminopeptidase B, aminopeptidase N, leucine aminopeptidase and the aminopeptidase activity of leukotriene A4 (LTA4) hydrolase. It is selective for these aminopeptidases over aminopeptidase A, trypsin, chymotrypsin, elastase, papain, pepsin and thermolysin. Immunomodulator. Displays immunostimulant activity via activation of macrophages and T lymphocytes. Displays anticancer and anti-inflammatory activity. Shown to inhibit proliferation, migration and invasion, and induces autophagy and apoptosis in cancer cells. Inhibits the production of LTB4 in erythrocytes. Has been used as useful tool to assess the physiological role of certain mammalian exopeptidases in the regulation of the immune system, the growth of tumors and their invasion of surrounding tissues and the degradation of cellular proteins.
Purity:
>98% (HPLC)
SMILES:
[NH3+][C@@H]([C@H](O)C(N[C@@H](CC(C)C)C(O)=O)=O)CC1=CC=CC=C1.[Cl-]
Solubility Chemicals:
Soluble in DMSO (10mg/ml), water (10mg/ml) or methanol (5mg/ml).
Source / Host:
Synthetic. Originally isolated from S. olivoreticuli.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20?C.
References
Bestatin, an inhibitor of aminopeptidase B, produced by actinomycetes: H. Umezawa, et al.; J. Antibiot. 29, 97 (1976) | Low-molecular-weight enzyme inhibitors of microbial origin: H. Umezawa; Ann. Rev. Microbiol. 36, 75 (1982) | Properties and specificity of binding sites for the immunomodulator bestatin on the surface of mammalian cells: W.E. Muller, et al.; Int. J. Immunopharmacol. 4, 393 (1982) | Inhibition of aminopeptidases by amastatin and bestatin derivatives. Effect of inhibitor structure on slow-binding processes: D.H. Rich, et al.; J. Med. Chem. 27, 417 (1984) | The slow, tight binding of bestatin and amastatin to aminopeptidases: S.H. Wilkes & J.M. Prescott; J. Biol. Chem. 260, 13154 (1985) | Leukotriene A4 hydrolase. Inhibition by bestatin and intrinsic aminopeptidase activity establish its functional resemblance to metallohydrolase enzymes: L. Orning, et al.; J. Biol. Chem. 266, 1375 (1991) | Bestatin, an aminopeptidase inhibitor with a multi-pharmacological function: G. Mathe; Biomed. Pharmacother. 45, 49 (1991) (Review) | Review of ubenimex (Bestatin): clinical research: K. Ota; Biomed. Pharmacother. 45, 55 (1991) (Review) | Inhibitory effect of bestatin on the growth of human lymphocytes: K. Ino, et al.; Immunopharmacol. 23, 163 (1992) | Inhibition of aminopeptidases N, A and W. A re-evaluation of the actions of bestatin and inhibitors of angiotensin converting enzyme: S. Tieku & N.M. Hooper; Biochem. Pharmacol. 44, 1725 (1992) | Clinical trials of bestatin for leukemia and solid tumors: K. Ota & Y. Uzuka; Biotherapy 4, 205 (1992) (Review) | Induction of apoptosis by bestatin (ubenimex) in human leukemic cell lines: K. Sekine, et al.; Leukemia 13, 729 (1999) | Anti-tumor angiogenesis effect of aminopeptidase inhibitor bestatin against B16-BL6 melanoma cells orthotopically implanted into syngeneic mice: Y. Aozuka, et al.; Cancer Lett. 216, 35 (2004) | Bestatin, an inhibitor for aminopeptidases, modulates the production of cytokines and chemokines by activated monocytes and macrophages: B. Lkhagvaa, et al.; Cytokine 44, 386 (2008) | The Analgesic Activity of Bestatin as a Potent APN Inhibitor: M.R. Jia, et al.; Front. Neurosci. 4, 50 (2010) (Review) | Ubenimex, an APN inhibitor, could serve as an anti?tumor drug in RT112 and 5637 cells by operating in an Akt?associated manner: X. Wang, et al.; Mol. Med. Rep. 17, 4531 (2018) | Inhibition of LTA4H by bestatin in human and mouse colorectal cancer: S. Zhao, et al.; EBioMedicine 44, 361 (2019)