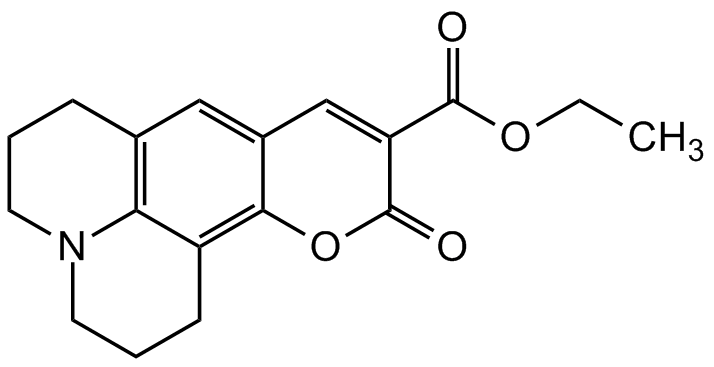
Coumarin 314
| Code | Size | Price |
|---|
| CDX-C0076-M500 | 500 mg | £89.00 |
Quantity:
| CDX-C0076-G001 | 1 g | £145.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
Short Term Storage: +20?C. Long Term Storage: +20?C
Images
Documents
Further Information
Alternate Names/Synonyms:
C314; Coumarin 504; NSC 338967; 2,3,5,6-1H,4H-Tetrahydro-9-carbethoxyquinolizino-[9,9a,1-gh]coumarin
Appearance:
Yellow to orange solid.
CAS:
55804-66-5
EClass:
32160000
Form (Short):
solid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C18H19NO4/c1-2-22-17(20)14-10-12-9-11-5-3-7-19-8-4-6-13(15(11)19)16(12)23-18(14)21/h9-10H,2-8H2,1H3
InChiKey:
VMJKUPWQKZFFCX-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 55804-66-5. Formula: C18H19NO4. Molecular Weight: 313.35. Coumarin 314 is a rigidized aminocoumarin derivative used as a laser dye with an absorption maximum of 436nm. It can be used as starting material for the synthesis of fluorescent probes. Spectral data: lambdamax 436nm in ethanol, epsilon (extinction coefficient) >39000 at 389-401 nm in ethanol, lambdalaser 504nm.
MDL:
MFCD00051334
Molecular Formula:
C18H19NO4
Molecular Weight:
313.35
Package Type:
Vial
Product Description:
Coumarin 314 is a rigidized aminocoumarin derivative used as a laser dye with an absorption maximum of 436nm. It can be used as starting material for the synthesis of fluorescent probes. Spectral data: lambdamax 436nm in ethanol, epsilon (extinction coefficient) >39000 at 389-401 nm in ethanol, lambdalaser 504nm.
Purity:
>98% (HPLC)
SMILES:
O=C1OC2=C3C4=C(CCCN4CCC3)C=C2C=C1C(OCC)=O
Solubility Chemicals:
Soluble in ethanol or DMSO.
Transportation:
Non-hazardous
UNSPSC Category:
Fluorescent Reagents
UNSPSC Number:
41105331
Use & Stability:
Stable for at least 2 years after receipt when stored at RT.
References
(1) G.A.Reynolds & K.H.Drexhage; Optics Commun. 13, 222 (1975) | (2) K.H.Drexhage; J. Res. Nati. Bureau Standards Sec. 1 - Phys. Chem. 80A, 421 (1976) | (3) A.N. Fletcher; Appl. Phys. 14, 295 (1977) | (4) A.J. Cox & B.K. Matise; Chem. Phys. Lett. 76, 125 (1980) | (5) A.N. Fletcher; Optics Commun. 48, 352 (1984) | (6) S. Speiser & N. Shakkour; Appl. Phys. B 38, 191 (1985) | (7) M.S.A. Abdel-Mottaleb; J. Photochem. Photobiol. A Chem. 50, 259 (1989) | (8) D.A. Pantano & D. Laria; J. Phys. Chem. B 107, 2971 (2003) | (9) A. Aspee, et al.; J. Phys. Chem. A 116, 199 (2012)