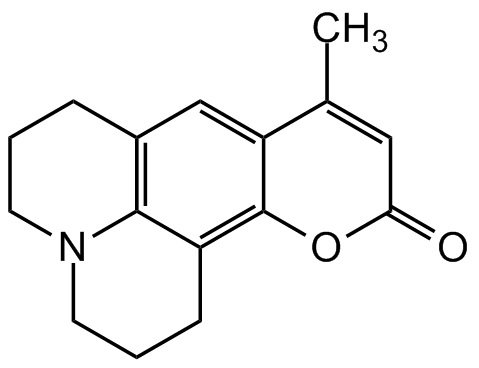
Coumarin 102
| Code | Size | Price |
|---|
| CDX-C0096-M250 | 250 mg | £48.00 |
Quantity:
| CDX-C0096-G001 | 1 g | £121.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
Short Term Storage: +20?C. Long Term Storage: +20?C
Images
Documents
Further Information
Alternate Names/Synonyms:
C102; Coumarin 480; NSC 290431; 2,3,6,7-Tetrahydro-9-methyl-1H,5H-quinolizino(9,1-gh)coumarin, 8-Methyl-2,3,5,6-tetrahydro-1H,4H-11-oxa-3a-aza-benzo(de)anthracen-10-one
Appearance:
Yellow solid.
CAS:
41267-76-9
EClass:
32160000
Form (Short):
solid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C16H17NO2/c1-10-8-14(18)19-16-12-5-3-7-17-6-2-4-11(15(12)17)9-13(10)16/h8-9H,2-7H2,1H3
InChiKey:
XHXMPURWMSJENN-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 41267-76-9. Formula: C16H17NO2. Molecular Weight: 255.31. Coumarin 102 also known as coumarin 480, is a laser dye and is widely used as a dynamic fluorescent probe of both small molecules and host cavities, micelles, polymers and solids. Coumarin 102 belongs to 7-aminocoumarins with a structurally rigidized amino group. Spectral data: lambdaex 390nm, lambdaem 466nm in ethanol, lambdalaser 480nm (461-513nm range).
MDL:
MFCD00041844
Molecular Formula:
C16H17NO2
Molecular Weight:
255.31
Package Type:
Vial
Product Description:
Coumarin 102 also known as coumarin 480, is a laser dye and is widely used as a dynamic fluorescent probe of both small molecules and host cavities, micelles, polymers and solids. Coumarin 102 belongs to 7-aminocoumarins with a structurally rigidized amino group. Spectral data: lambdaex 390nm, lambdaem 466nm in ethanol, lambdalaser 480nm (461-513nm range).
Purity:
>98% (HPLC)
SMILES:
O=C1OC2=C3C4=C(CCCN4CCC3)C=C2C(C)=C1
Solubility Chemicals:
Soluble in ethanol or DMSO.
Transportation:
Non-hazardous
UNSPSC Category:
Fluorescent Reagents
UNSPSC Number:
41105331
Use & Stability:
Stable for at least 2 years after receipt when stored at RT.
References
(1) G.A. Reynolds & K.H. Drexhage; Optics Commun. 13, 222 (1975) | (2) D. Huppert & P.M. Rentzepis; J. Appl. Phys. 49, 543 (1978) | (3) A.J. Campillo, et al.; Chem. Phys. Lett. 67, 218 (1979) | (4) S.L. Shapiro & K.R. Winn; Chem. Phys. Lett. 71, 440 (1980) | (5) J.T. Kunjappu, et al.; J. Photochem. Photobiol. A: Chemistry 71, 269 (1993) | (6) K. Das, et al.; Chem. Phys. Lett. 249, 323 (1996) | (7) N. Sarkar, et al.; J. Phys. Chem. 100, 15483 (1996) | (8) P. Sen, et al.; Chem. Phys. Lett. 411, 339 (2005) | (9) T. Sen, et al.; J. Phys. Chem. C 114, 11409 (2010) | (10) G.-Y. Liu, et al.; J. Mat. Chem. 22, 16865 (2012) | (11) E.S. Savenkoa & V.V. Kostjukov; New J. Chem. 46, 2441 (2022)