Enocitabine
| Code | Size | Price |
|---|
| TAR-T15235-1mg | 1mg | £195.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T15235-5mg | 5mg | £334.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T15235-10mg | 10mg | £523.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T15235-25mg | 25mg | £785.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T15235-50mg | 50mg | £1,018.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T15235-100mg | 100mg | £1,348.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
cool pack
Storage:
-20℃
Images
Documents
Further Information
Bioactivity:
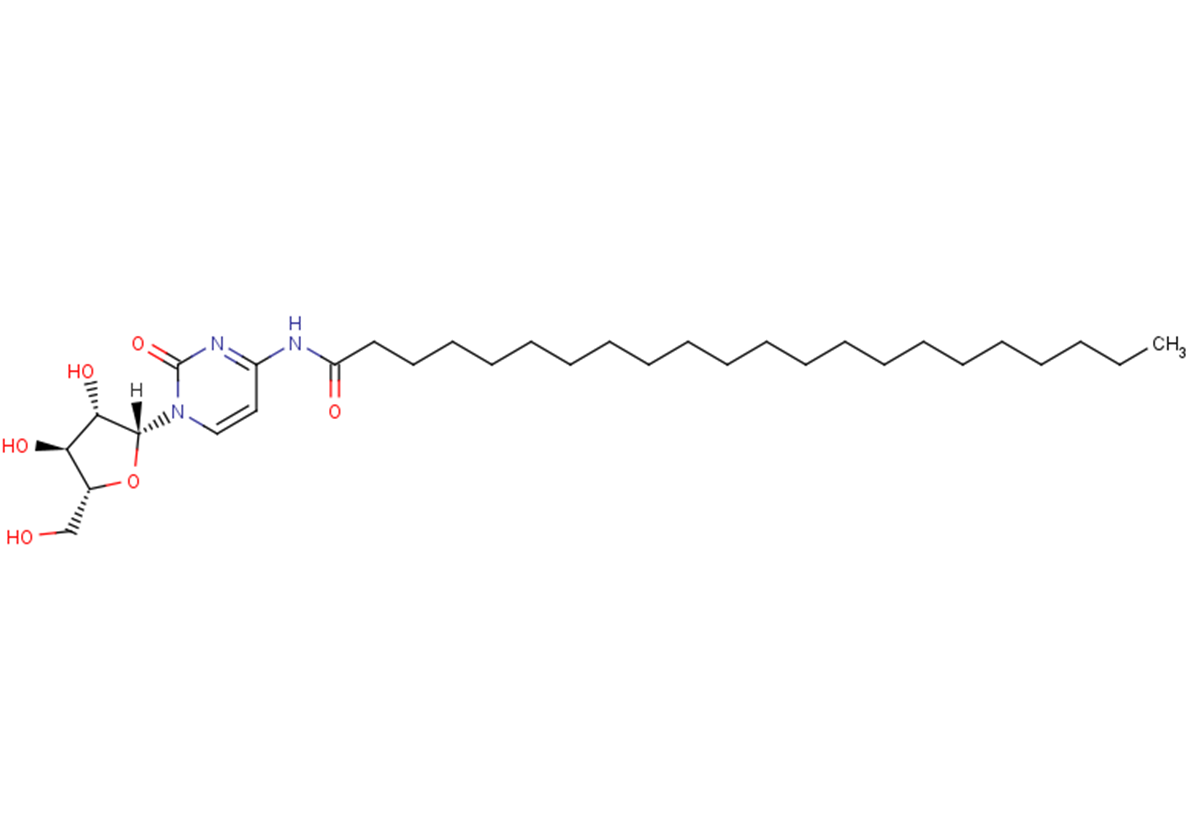
Enocitabine is a nucleoside analog. Enocitabine inhibits the replication of human cytomegalovirus(HCMV) and it also has antileukemic and antiviral activities. Enocitabine is also a potent DNA replication inhibitor and a DNA chain terminator.
CAS:
55726-47-1
Formula:
C31H55N3O6
Molecular Weight:
565.796
Pathway:
DNA Damage/DNA Repair; Proteases/Proteasome; Microbiology/Virology; Cell Cycle/Checkpoint
Purity:
0.98
SMILES:
CCCCCCCCCCCCCCCCCCCCCC(=O)Nc1ccn([C@@H]2O[C@H](CO)[C@@H](O)[C@@H]2O)c(=O)n1
Target:
Nucleoside Antimetabolite/Analog; HCV Protease; DNA/RNA Synthesis
References
1. Hamada A, et al. Clinical pharmacokinetics of cytarabine formulations.Clin Pharmacokinet. 2002;41(10):705-18.
2. Nagasawa M, et al.In vitro combined effects of pirarubicin (THP) and various antitumor drugs on human tumor cell lines. Gan To Kagaku Ryoho. 1990 Apr;17(4 Pt 1):633-8.
3. Nakamura K, et al. Antiviral effect of antileukemic drugs N4-behenoyl-1-beta-D-arabinofuranosylcytosine (BH-AC) and 2,2'-anhydro-1-beta-D-arabinofuranosylcytosine (cyclo-C) against human cytomegalovirus. J Med Virol. 1990 Jun;31(2):141-7.