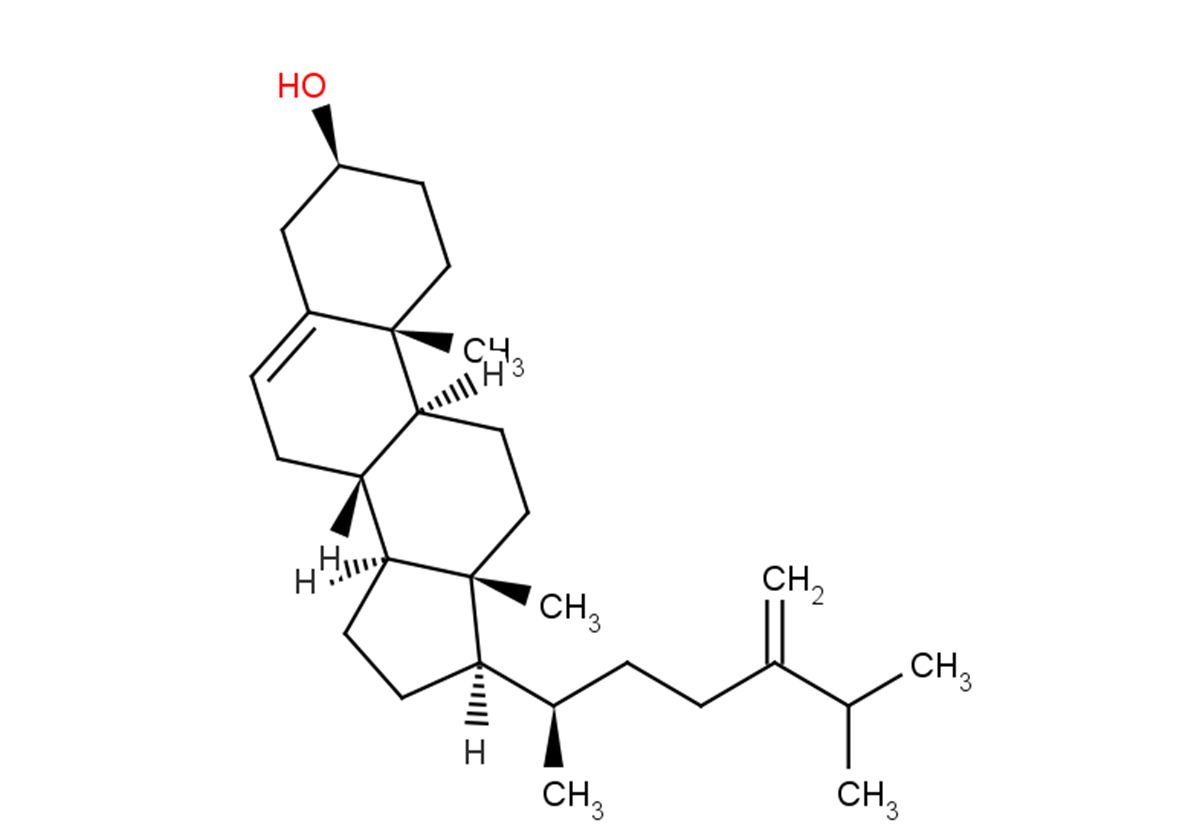
24-Methylenecholesterol
| Code | Size | Price |
|---|
| TAR-T29329-10mg | 10mg | Enquire | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T29329-5mg | 5mg | Enquire | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T29329-1mg | 1mg | £1,049.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
cool pack
Storage:
-20℃
Images
Documents
Further Information
Bioactivity:
24-Methylenecholesterol is a natural marine sterol, which stimulates cholesterol acyl transferase (ACAT) in human macrophages.
CAS:
474-63-5
Formula:
C28H46O
Molecular Weight:
398.66
Pathway:
Metabolism
Purity:
0.98
SMILES:
C[C@@]12[C@]([C@]3([C@@]([C@]4(C)C(=CC3)C[C@@H](O)CC4)(CC1)[H])[H])(CC[C@@]2([C@@H](CCC(C(C)C)=C)C)[H])[H]
Target:
Acyltransferase
References
1. Tsukagoshi Y, et al. Ajuga ?24-Sterol Reductase Catalyzes the Direct Reductive Conversion of 24-Methylenecholesterol to Campesterol. J Biol Chem. 2016 Apr 8;291(15):8189-98.
2. Nasu K, et al. Stereochemical fate of C-26 and C-27 during the conversion of isofucosterol to sitosterol and of 24-methylenecholesterol to campesterol and dihydrobrassicasterol in Oryza sativa cell cultures. Phytochemistry. 2000 Jun;54(4):381-5.
3. Choe S, et al. The Arabidopsis dwarf1 mutant is defective in the conversion of 24-methylenecholesterol to campesterol in brassinosteroid biosynthesis. Plant Physiol. 1999 Mar;119(3):897-907.
4. Barbier M. Isolation of 24-methylenecholesterol-derived oxidation products from queen honeybee ovaries (Apis mellifica L.). J Chem Ecol. 1987 Jul;13(7):1681-7.