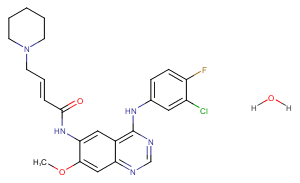
Dacomitinib hydrate
| Code | Size | Price |
|---|
| TAR-T19965-1mg | 1mg | £119.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T19965-2mg | 2mg | £140.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T19965-5mg | 5mg | £177.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T19965-1mL | 1 mL * 10 mM (in DMSO) | £206.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T19965-10mg | 10mg | £226.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T19965-25mg | 25mg | £340.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T19965-50mg | 50mg | £475.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T19965-100mg | 100mg | £658.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T19965-500mg | 500mg | £1,299.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
cool pack
Storage:
-20℃
Images
Documents
Further Information
Bioactivity:
Dacomitinib hydrate is a highly selective and second-generation small-molecule inhibitor of the pan-epidermal growth factor receptor family of tyrosine kinases. Dacomitinib specifically and irreversibly binds to and inhibits human EGFR subtypes, resulting in inhibition of proliferation and induction of apoptosis in EGFR-expressing tumor cells.
CAS:
1042385-75-0
Formula:
C24H27ClFN5O3
Molecular Weight:
487.96
Pathway:
Angiogenesis; Tyrosine Kinase/Adaptors; JAK/STAT signaling
Purity:
0.9805
SMILES:
O.COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1NC(=O)C=CCN1CCCCC1
Target:
EGFR
References
1. Ou SH, Soo RA. Dacomitinib in lung cancer: a "lost generation" EGFR tyrosine-kinase inhibitor from a bygone era? Drug Des Devel Ther. 2015 Oct 15;9:5641-53. doi: 10.2147/DDDT.S52787. eCollection 2015. Review. PubMed PMID: 26508839.
2. Ramalingam SS, J?nne PA, Mok T, O'Byrne K, Boyer MJ, Von Pawel J, Pluzanski A, Shtivelband M, Docampo LI, Bennouna J, Zhang H, Liang JQ, Doherty JP, Taylor I, Mather CB, Goldberg Z, O'Connell J, Paz-Ares L. Dacomitinib versus erlotinib in patients with advanced-stage, previously treated non-small-cell lung cancer (ARCHER 1009): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2014 Nov;15(12):1369-78. doi: 10.1016/S1470-2045(14)70452-8. Epub 2014 Oct 15. PubMed PMID: 25439691.
3. Giri N, Masters JC, Plotka A, Liang Y, Boutros T, Pardo P, O'Connell J, Bello C. Investigation of the impact of hepatic impairment on the pharmacokinetics of dacomitinib. Invest New Drugs. 2015 Aug;33(4):931-41. doi: 10.1007/s10637-015-0256-0. Epub 2015 Jun 6. PubMed PMID: 26048096.
4. Ellis PM, Shepherd FA, Millward M, et al. Dacomitinib compared with placebo in pretreated patients with advanced or metastatic non-small-cell lung cancer (NCIC CTG BR.26): a double-blind, randomised, phase 3 trial. Lancet Oncol. 2014 Nov;15(12):1379-88. doi: 10.1016/S1470-2045(14)70472-3. Epub 2014 Oct 15. PubMed PMID: 25439692.