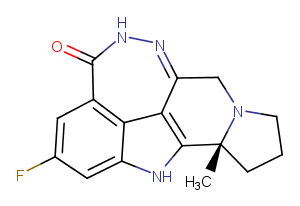
Pamiparib
| Code | Size | Price |
|---|
| TAR-T5058-10mg | 10mg | Enquire | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T5058-50mg | 50mg | Enquire | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T5058-1mg | 1mg | £95.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T5058-2mg | 2mg | £103.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T5058-5mg | 5mg | £111.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T5058-1mL | 1 mL * 10 mM (in DMSO) | £117.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
cool pack
Storage:
-20℃
Images
Documents
Further Information
Bioactivity:
BGB-290 is an orally active, potent, highly selective PARP inhibitor(IC50 of 0.9 nM and 0.5 nM for PARP1 and PARP2, respectively). It has potent PARP trapping, and capability to penetrate the brain, and can be used for the research of various cancers including the solid tumor
CAS:
1446261-44-4
Formula:
C16H15FN4O
Molecular Weight:
298.321
Pathway:
Chromatin/Epigenetic; DNA Damage/DNA Repair
Purity:
0.9953
SMILES:
C[C@]12CCCN1Cc1n[nH]c(=O)c3cc(F)cc4[nH]c2c1c34
Target:
PARP
References
Friedlander M, et al. Pamiparib in combination with tislelizumab in patients with advanced solid tumours: results from the dose-escalation stage of a multicentre, open-label, phase 1a/b trial. Lancet Oncol. 2019 Sep;20(9):1306-1315.
Shiv K. Gupta, et al. Abstract 3505: Inhibition of PARP activity by BGB-290 potentiates efficacy of NSC 362856 in patient derived xenografts of glioblastoma multiforme. Cancer Research. August 2015, Volume 75, Issue 15
Xiong Y , Guo Y , Liu Y , et al. Pamiparib is a potent and selective PARP inhibitor with unique potential for the treatment of brain tumor[J]. Neoplasia (New York, N.Y.), 2020, 22(9):431-440.