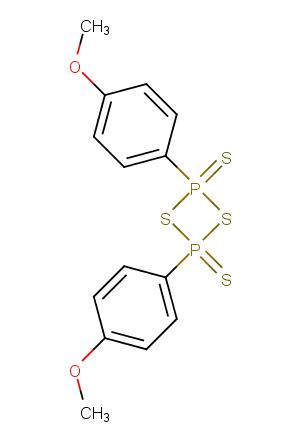
Lawesson's reagent
| Code | Size | Price |
|---|
| TAR-T20652-5mg | 5mg | £850.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
cool pack
Storage:
-20℃
Images
Documents
Further Information
Bioactivity:
Lawesson's reagent is a thiation agent. It is incompatible with strong oxidizing agents.
CAS:
19172-47-5
Formula:
C14H14O2P2S4
Molecular Weight:
404.45
Purity:
0.98
SMILES:
COc1ccc(cc1)P1(=S)SP(=S)(S1)c1ccc(OC)cc1
References
1. Nicolau LA, Silva RO, Damasceno SR, Carvalho NS, Costa NR, Arag?o KS, Barbosa AL, Soares PM, Souza MH, Medeiros JV. The hydrogen sulfide donor, Lawesson's reagent, prevents alendronate-induced gastric damage in rats. Braz J Med Biol Res. 2013 Aug;46(8):708-14. doi: 10.1590/1414-431X20133030. Epub 2013 Aug 16. PubMed PMID: 23969974; PubMed Central PMCID: PMC3854416.
2. Kumar SV, Parameshwarappa G, Ila H. Synthesis of 2,4,5-trisubstituted thiazoles via Lawesson's reagent-mediated chemoselective thionation-cyclization of functionalized enamides. J Org Chem. 2013 Jul 19;78(14):7362-9. doi: 10.1021/jo401208u. Epub 2013 Jul 10. PubMed PMID: 23815778.
3. Huang P, Zhang R, Liang Y, Dong D. Lawesson's reagent-initiated domino reaction of aminopropenoyl cyclopropanes: synthesis of thieno[3,2-c]pyridinones. Org Biomol Chem. 2012 Feb 28;10(8):1639-44. doi: 10.1039/c2ob06709a. Epub 2012 Jan 11. PubMed PMID: 22234526.
4. Qiu G, Hu Y, Ding Q, Peng Y, Hu X, Wu J. Synthesis of 4-methylene-4H-benzo[d][1,3]thiazines via a tandem reaction of 1-(2-alkynylphenyl)ketoximes with Lawesson's reagent. Chem Commun (Camb). 2011 Sep 14;47(34):9708-10. doi: 10.1039/c1cc12937f. Epub 2011 Jul 27. PubMed PMID: 21792441.