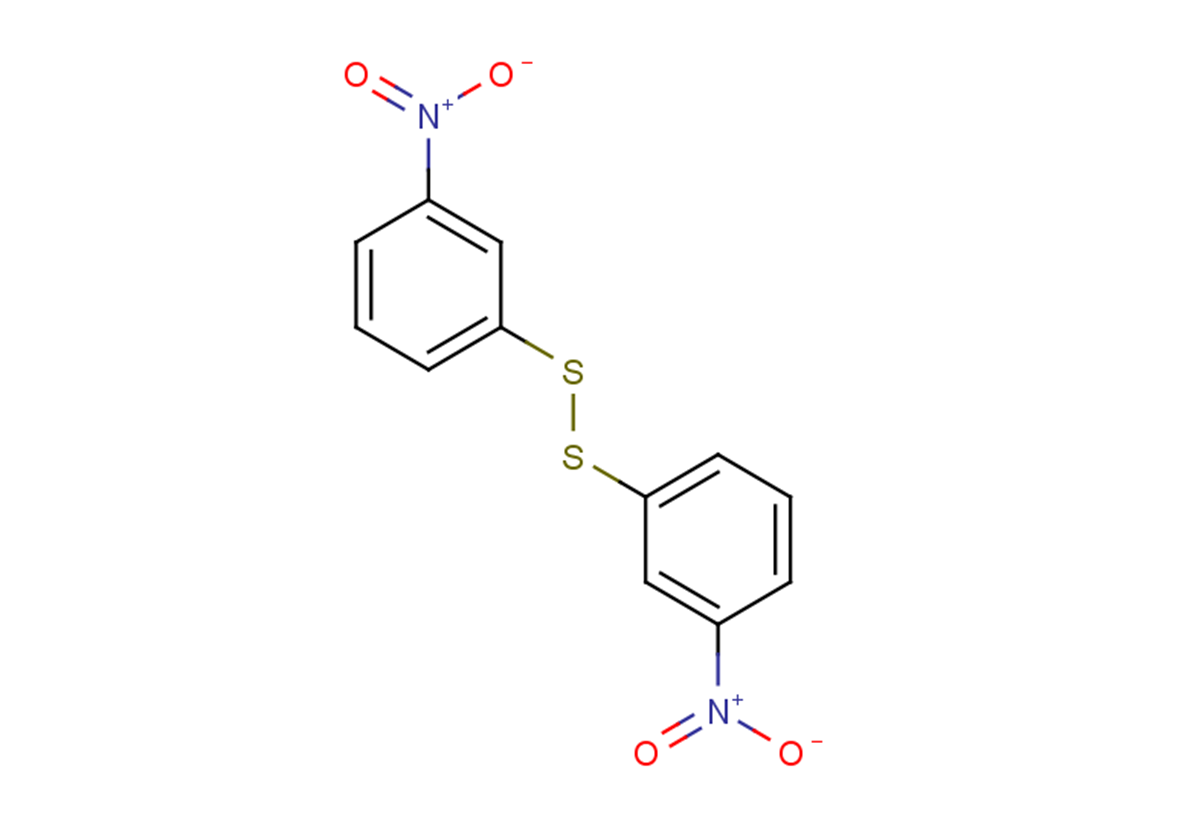
Nitrophenide
| Code | Size | Price |
|---|
| TAR-T21089-5mg | 5mg | £111.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T21089-10mg | 10mg | £127.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T21089-25mg | 25mg | £162.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T21089-50mg | 50mg | £205.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T21089-100mg | 100mg | £270.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T21089-500mg | 500mg | £500.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
cool pack
Storage:
-20℃
Images
Documents
Further Information
Bioactivity:
Nitrophenide inhibits mannitol-1-phosphate dehydrogenase (M1PDH), which catalyzes the committed enzymatic step in the mannitol cycle. Nitrophenide can be used as an anticoccidial agent.
CAS:
537-91-7
Formula:
C12H8N2O4S2
Molecular Weight:
308.33
Pathway:
Metabolism
Purity:
0.9943
SMILES:
[O-][N+](=O)c1cccc(SSc2cccc(c2)[N+]([O-])=O)c1
Target:
Dehydrogenase
References
1. Allocco JJ, et al. Nitrophenide (Megasul) blocks Eimeria tenella development by inhibiting the mannitol cycle enzyme mannitol-1-phosphate dehydrogenase. J Parasitol. 2001 Dec;87(6):1441-8.
2. Zimnicka M, et al. Reactions of nitrophenide and halonitrophenide ions with acrylonitrile and alkyl acrylates in the gas phase: addition to the carbonyl group versus Michael addition. J Mass Spectrom. 2012 Apr;47(4):425-38.
3. Danikiewicz W, et al. Aromatic nucleophilic substitution (SNAr) reactions of 1,2- and 1,4-halonitrobenzenes and 1,4-dinitrobenzene with carbanions in the gas phase. J Am Soc Mass Spectrom. 2007 Aug;18(8):1351-63. Epub 2007 Apr 25.
4. Danikiewicz W, Bie?kowski T, Poddebniak D. Generation and reactions of anionic sigma-adducts of 1,3-dinitrobenzene and 1,3,5-trinitrobenzene with carbanions in a gas phase, using an electrospray ion source as the chemical reactor. J Am Soc Mass Spectrom. 2004 Jun;15(6):927-33.