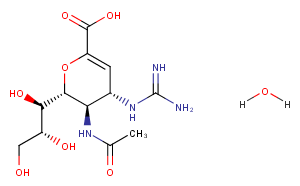
Zanamivir hydrate
| Code | Size | Price |
|---|
| TAR-T2529L-5mg | 5mg | £850.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
cool pack
Storage:
-20℃
Images
Documents
Further Information
Bioactivity:
Zanamivir hydrate is neuraminidase inhibitors. It is used worldwide for the treatment and prophylaxis of influenza caused by influenza A and B viruses.
CAS:
551942-41-7
Formula:
C12H22N4O8
Molecular Weight:
350.328
Purity:
0.98
SMILES:
O.CC(=O)N[C@@H]1[C@@H](NC(N)=N)C=C(O[C@H]1[C@H](O)[C@H](O)CO)C(O)=O
References
1. Heneghan CJ, Onakpoya I, Thompson M, Spencer EA, Jones M, Jefferson T. Zanamivir for influenza in adults and children: systematic review of clinical study reports and summary of regulatory comments. BMJ. 2014 Apr 9;348:g2547. doi: 10.1136/bmj.g2547. Review. PubMed PMID: 24811412; PubMed Central PMCID: PMC3981976.
2. Pizzorno A, Abed Y, Rh?aume C, Boivin G. Oseltamivir-zanamivir combination therapy is not superior to zanamivir monotherapy in mice infected with influenza A(H3N2) and A(H1N1)pdm09 viruses. Antiviral Res. 2014 May;105:54-8. doi: 10.1016/j.antiviral.2014.02.017. Epub 2014 Feb 28. PubMed PMID: 24583158.
3. Chan-Tack KM, Kim C, Moruf A, Birnkrant DB. Clinical experience with intravenous zanamivir under an Emergency IND program in the United States (2011-2014). Antivir Ther. 2015;20(5):561-4. doi: 10.3851/IMP2944. Epub 2015 Feb 10. Review. PubMed PMID: 25667992.
4. Dunstan HJ, Mill AC, Stephens S, Yates LM, Thomas SH. Pregnancy outcome following maternal use of zanamivir or oseltamivir during the 2009 influenza A/H1N1 pandemic: a national prospective surveillance study. BJOG. 2014 Jun;121(7):901-6. doi: 10.1111/1471-0528.12640. Epub 2014 Mar 7. PubMed PMID: 24602087.