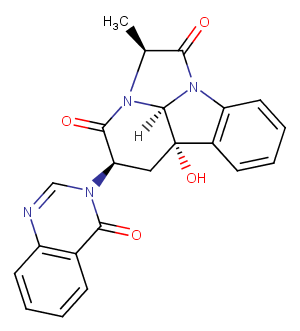
Chaetominine
| Code | Size | Price |
|---|
| TAR-T30869-5mg | 5mg | £2,162.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
cool pack
Storage:
-20℃
Images
Documents
Further Information
Bioactivity:
Chaetominine is a type of cytotoxic alkaloid obtained from endophytic Chaetomium sp. IFB-E015.
CAS:
918659-56-0
Formula:
C22H18N4O4
Molecular Weight:
402.41
Purity:
0.98
SMILES:
[H][C@]12N3[C@@H](C)C(=O)N1c1ccccc1[C@@]2(O)C[C@H](C3=O)n1cnc2ccccc2c1=O
References
1. Liu CQ, Pan ZH, An FL, Lu YH. Co-addition Strategy for Enhancement of Chaetominine from Submerged Fermentation of Aspergillus fumigatus CY018. Appl Biochem Biotechnol. 2018 Apr 10. doi: 10.1007/s12010-018-2714-6. [Epub ahead of print] PubMed PMID: 29637396.
2. Zhou Y, Breit B. Rhodium-Catalyzed Asymmetric N-H Functionalization of Quinazolinones with Allenes and Allylic Carbonates: The First Enantioselective Formal Total Synthesis of (-)-Chaetominine. Chemistry. 2017 Dec 22;23(72):18156-18160. doi: 10.1002/chem.201705059. Epub 2017 Dec 5. PubMed PMID: 29105185.
3. Yao J, Wei X, Lu Y. Chaetominine reduces MRP1-mediated drug resistance via inhibiting PI3K/Akt/Nrf2 signaling pathway in K562/Adr human leukemia cells. Biochem Biophys Res Commun. 2016 May 13;473(4):867-873. doi: 10.1016/j.bbrc.2016.03.141. Epub 2016 Mar 30. PubMed PMID: 27038543.
4. Liu C, Jiao R, Yao L, Zhang Y, Lu Y, Tan R. Adsorption characteristics and preparative separation of chaetominine from Aspergillus fumigatus mycelia by macroporous resin. J Chromatogr B Analyt Technol Biomed Life Sci. 2016 Mar 15;1015-1016:135-141. doi: 10.1016/j.jchromb.2016.02.027. Epub 2016 Feb 20. PubMed PMID: 26919448.