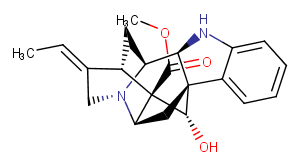
Quebrachidine
| Code | Size | Price |
|---|
| TAR-T34220-5mg | 5mg | £850.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
cool pack
Storage:
-20℃
Images
Documents
Further Information
Bioactivity:
Quebrachidine is a biochemical.
CAS:
4835-69-2
Formula:
C21H24N2O3
Molecular Weight:
352.434
Purity:
0.98
SMILES:
COC(=O)[C@@]12[C@H]3C[C@]4([C@H](Nc5ccccc45)[C@@H]4C[C@H]1C(CN34)=CC)[C@@H]2O
References
Yu J, Wearing XZ, Cook JM. A general strategy for the synthesis of vincamajine-related indole alkaloids: stereocontrolled total synthesis of (+)-dehydrovoachalotine, (-)-vincamajinine, and (-)-11-methoxy-17-epivincamajine as well as the related quebrachidine diol, vincamajine diol, and vincarinol. J Org Chem. 2005 May 13;70(10):3963-79. PubMed PMID: 15876085.
Aynilian GH, Bell CL, Farnsworth NR. Alkaloids of Vinca species V: structure elucidation of herbadine, an alkaloid isolated from Vinca libanotica. J Pharm Sci. 1975 Feb;64(2):341-2. PubMed PMID: 1127594.
Yin W, Ma J, Rivas FM, Cook JM. First enantiospecific total synthesis of the important biogenetic intermediates, (+)-polyneuridine and (+)-polyneuridine aldehyde, as well as 16-epi-vellosimine and macusine A. Org Lett. 2007 Jan 18;9(2):295-8. PubMed PMID: 17217288.
Yin W, Kabir MS, Wang Z, Rallapalli SK, Ma J, Cook JM. Enantiospecific total synthesis of the important biogenetic intermediates along the ajmaline pathway, (+)-polyneuridine and (+)-polyneuridine aldehyde, as well as 16-epivellosimine and macusine A. J Org Chem. 2010 May 21;75(10):3339-49. doi: 10.1021/jo100279w. PubMed PMID: 20392128; PubMed Central PMCID: PMC3188852.