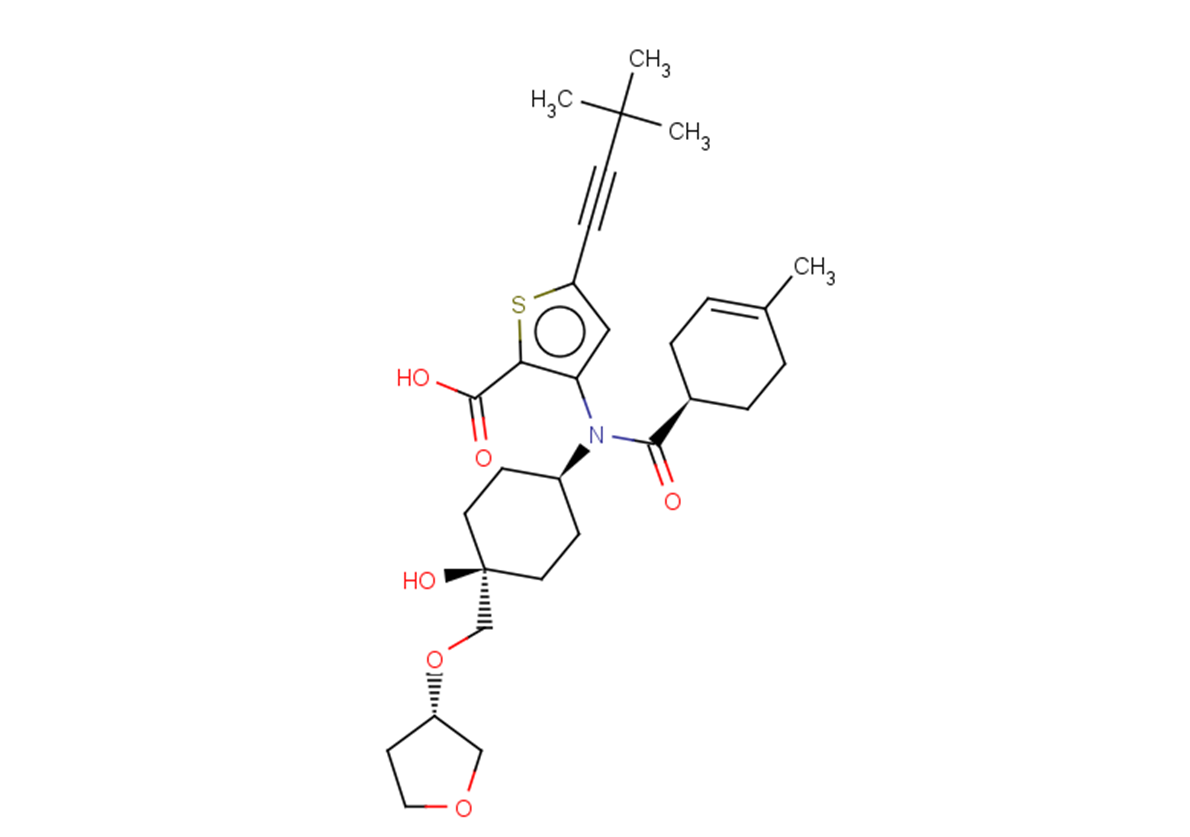
Radalbuvir
| Code | Size | Price |
|---|
| TAR-T34254-5mg | 5mg | £1,251.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T34254-50mg | 50mg | £2,464.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T34254-100mg | 100mg | £3,283.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
cool pack
Storage:
-20℃
Images
Documents
Further Information
Bioactivity:
Radalbuvir (GS-9669) is an investigational antiviral agent for the treatment of hepatitis C virus (HCV) infection as an NS5B inhibitor.
CAS:
1314795-11-3
Formula:
C30H41NO6S
Molecular Weight:
543.72
Purity:
0.98
SMILES:
CC1=CC[C@@H](CC1)C(=O)N([C@H]1CC[C@@](O)(CO[C@H]2CCOC2)CC1)c1cc(sc1C(O)=O)C#CC(C)(C)C
References
Kohli A, Kattakuzhy S, Sidharthan S, Nelson A, McLaughlin M, Seamon C, Wilson E, Meissner EG, Sims Z, Silk R, Gross C, Akoth E, Tang L, Price A, Jolley TA, Emmanuel B, Proschan M, Teferi G, Chavez J, Abbott S, Osinusi A, Mo H, Polis MA, Masur H, Kottilil S. Four-Week Direct-Acting Antiviral Regimens in Noncirrhotic Patients With Hepatitis C Virus Genotype 1 Infection: An Open-Label, Nonrandomized Trial. Ann Intern Med. 2015 Dec 15;163(12):899-907. doi: 10.7326/M15-0642. Epub 2015 Nov 24. PubMed PMID: 26595450.
Meissner EG, Kohli A, Virtaneva K, Sturdevant D, Martens C, Porcella SF, McHutchison JG, Masur H, Kottilil S. Achieving sustained virologic response after interferon-free hepatitis C virus treatment correlates with hepatic interferon gene expression changes independent of cirrhosis. J Viral Hepat. 2016 Jul;23(7):496-505. doi: 10.1111/jvh.12510. Epub 2016 Feb 3. PubMed PMID: 26840694.
Petersen T, Townsend K, Gordon LA, Sidharthan S, Silk R, Nelson A, Gross C, Calder?n M, Proschan M, Osinusi A, Polis MA, Masur H, Kottilil S, Kohli A. High adherence to all-oral directly acting antiviral HCV therapy among an inner-city patient population in a phase 2a study. Hepatol Int. 2016 Mar;10(2):310-9. doi: 10.1007/s12072-015-9680-7. Epub 2015 Nov 26. PubMed PMID: 26612014; PubMed Central PMCID: PMC4778154.
Lawitz E, Poordad F, Hyland RH, Wang J, Liu L, Dvory-Sobol H, Brainard DM, McHutchison JG, Gutierrez JA. Ledipasvir/sofosbuvir-based treatment of patients with chronic genotype-1 HCV infection and cirrhosis: results from two Phase II studies. Antivir Ther. 2016 Jun 27. doi: 10.3851/IMP3062. [Epub ahead of print] PubMed PMID: 27348483.