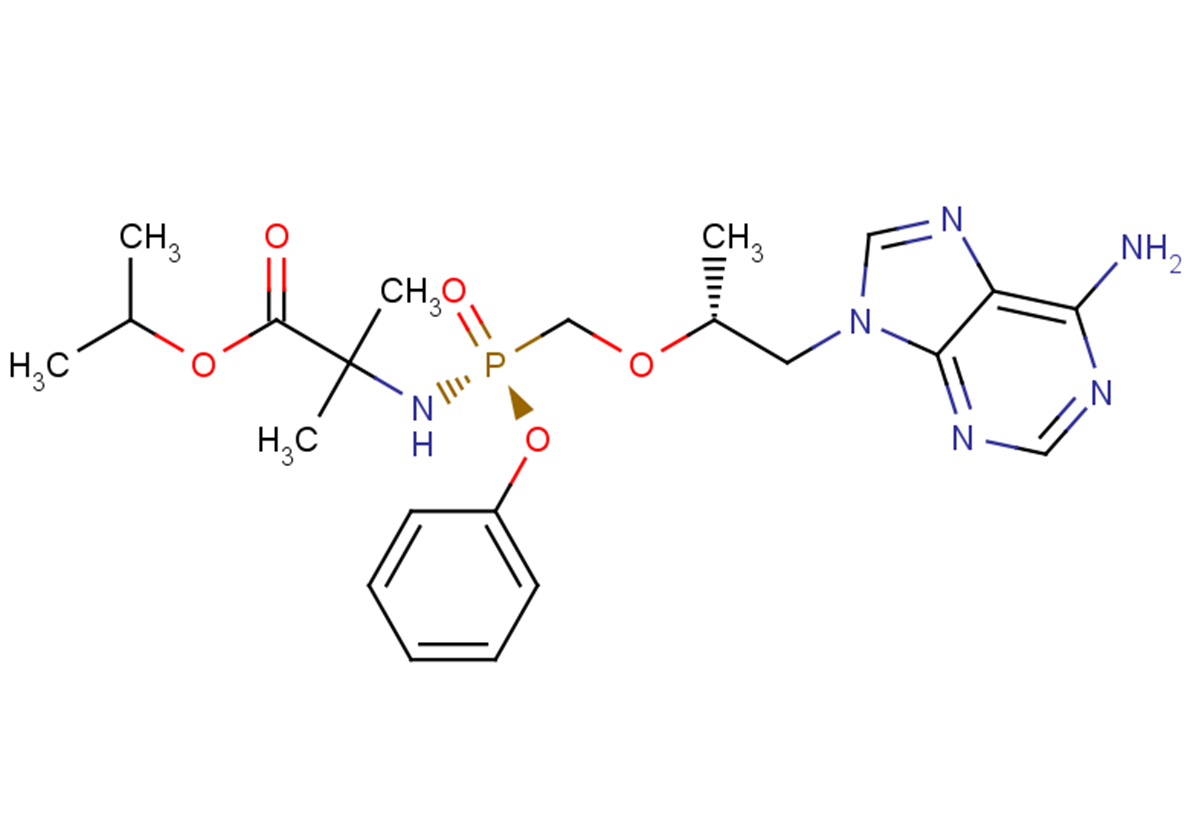
Tenofovir amibufenamide
| Code | Size | Price |
|---|
| TAR-T39043-1mg | 1mg | £306.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T39043-5mg | 5mg | £648.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T39043-10mg | 10mg | £863.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T39043-25mg | 25mg | £1,267.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T39043-50mg | 50mg | £1,661.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T39043-100mg | 100mg | £2,279.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
cool pack
Storage:
-20℃
Images
Documents
Further Information
Bioactivity:
Tenofovir amibufenamide (HS-10234), a Tenofovir prodrug, is an orally active antiviral agent. Tenofovir amibufenamide inhibits HBV , and can be used for chronic hepatitis B (CHB) study.
CAS:
1571076-26-0
Formula:
0
Molecular Weight:
0
Pathway:
Microbiology/Virology
Purity:
0.98
SMILES:
0
Target:
HBV
References
Liu Z, et al. Randomised clinical trial: 48 weeks of treatment with tenofovir amibufenamide versus tenofovir disoproxil fumarate for patients with chronic hepatitis B. Aliment Pharmacol Ther. 2021;54(9):1134-1149.
Hong X, et al. Improved pharmacokinetics of tenofovir ester prodrugs strengthened the inhibition of HBV replication and the rebalance of hepatocellular metabolism in preclinical models. Front Pharmacol. 2022;13:932-934.
Zhihong Liu, et al. Randomised clinical trial: 48 weeks of treatment with tenofovir amibufenamide versus tenofovir disoproxil fumarate for patients with chronic hepatitis B. Aliment Pharmacol Ther. 2021 Nov;54(9):1134-1149.
Hong Zhang, et al. Randomised clinical trial: safety, efficacy and pharmacokinetics of HS-10234 versus tenofovir for the treatment of chronic hepatitis B infection. Aliment Pharmacol Ther. 2021 Jan;53(2):243-252.