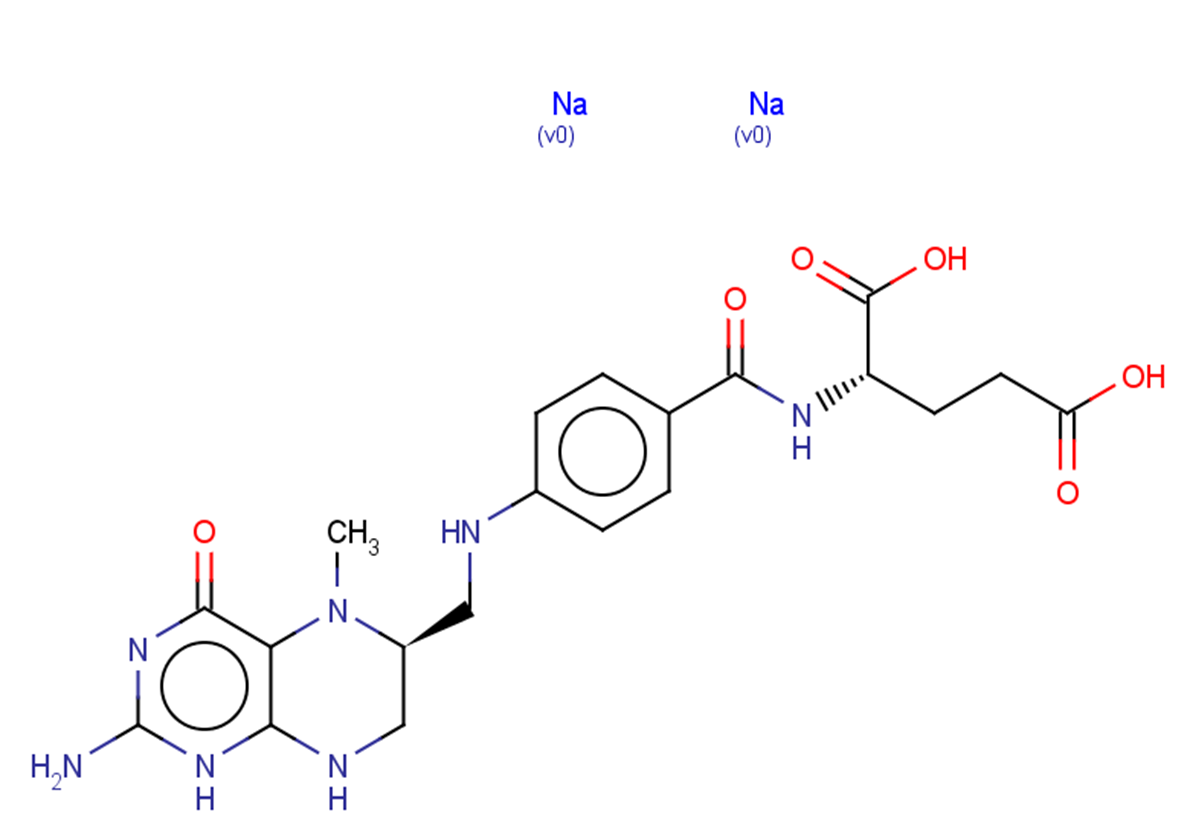
Levomefolate sodium
| Code | Size | Price |
|---|
| TAR-T7593L-5mg | 5mg | £850.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T7593L-50mg | 50mg | £1,661.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T7593L-100mg | 100mg | £2,079.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
cool pack
Storage:
-20℃
Images
Documents
Further Information
Bioactivity:
Levomefolate calcium is a primary biologically active form of folate used at the cellular level for dna reproduction, the cysteine cycle and the regulation of homocysteine
CAS:
1423663-76-6
Formula:
C20H23N7Na2O6
Molecular Weight:
503.43
Purity:
0.98
SMILES:
O=C(CC[C@H](NC(C1=CC=C(C=C1)NC[C@@H]2N(C3=C(N=C(N=C3NC2)N)O)C)=O)C([O-])=O)O[Na].[Na+]
References
Rapkin RB, Creinin MD. The combined oral contraceptive pill containing drospirenone and ethinyl estradiol plus levomefolate calcium. Expert Opin Pharmacother. 2011 Oct;12(15):2403-10. doi: 10.1517/14656566.2011.610791. Epub 2011 Aug 31. Review. PubMed PMID: 21877996.
Diefenbach K, Trummer D, Ebert F, Lissy M, Koch M, Rohde B, Blode H. EE-drospirenone-levomefolate calcium versus EE-drospirenone + folic acid: folate status during 24 weeks of treatment and over 20 weeks following treatment cessation. Int J Womens Health. 2013 Apr 11;5:149-63. doi: 10.2147/IJWH.S37254. Print 2013. PubMed PMID: 23610531; PubMed Central PMCID: PMC3628530.
Blode H, Klipping C, Richard F, Trummer D, Rohde B, Diefenbach K. Bioequivalence study of an oral contraceptive containing ethinylestradiol/drospirenone/levomefolate calcium relative to ethinylestradiol/drospirenone and to levomefolate calcium alone. Contraception. 2012 Feb;85(2):177-84. doi: 10.1016/j.contraception.2011.05.015. Epub 2011 Jul 19. PubMed PMID: 22067789.
Wiesinger H, Eydeler U, Richard F, Trummer D, Blode H, Rohde B, Diefenbach K. Bioequivalence evaluation of a folate-supplemented oral contraceptive containing ethinylestradiol/drospirenone/levomefolate calcium versus ethinylestradiol/drospirenone and levomefolate calcium alone. Clin Drug Investig. 2012 Oct 1;32(10):673-84. doi: 10.2165/11635280-000000000-00000. PubMed PMID: 22909145.