Deferoxamine Mesylate
| Code | Size | Price |
|---|
| TAR-T1637-100mg | 100mg | £111.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T1637-1mL | 1 mL * 10 mM (in DMSO) | £122.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
cool pack
Storage:
-20℃
Images
Documents
Further Information
Bioactivity:
Deferoxamine is an iron-chelating agent that binds free iron in a stable complex. It also is an inhibitor of ferroptosis.
Biological Applications:
Ferrostatin-1 and Deferoxamine mesylate, as inhibitors of ferroptosis, play crucial roles in regulating this iron-dependent form of cell death. Ferrostatin-1 regulates ferroptosis by influencing lipid peroxidation and iron metabolism, while Deferoxamine mesylate not only inhibits ferroptosis but also exhibits excellent antioxidant, anti-proliferative, and anti-tumor activities. Therefore, Deferoxamine mesylate is applicable to research in diabetes, neurodegenerative diseases, as well as in anti-cancer and anti-COVID-19 studies. Abundant research indicates that modulating ferroptosis is pivotal in tumor suppression, immunity, neurodegenerative diseases, tissue and organ damage, as well as various inflammatory and infectious diseases.
CAS:
138-14-7
Description:
Deferoxamine is an iron-chelating agent that binds free iron in a stable complex. It also is an inhibitor of ferroptosis.
Formula:
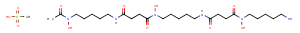
C26H52N6O11S
Mechanism of Action:
Ferroptosis is a form of regulated cell death primarily caused by iron-dependent oxidative damage. Under physiological conditions, iron levels in cells are in dynamic equilibrium. Excessive iron ions can lead to cellular iron overload, characterized by the deposition of iron ions causing lipid peroxidation reactions and the accumulation of reactive oxygen species (ROS) generated by iron metabolism. Ultimately, this results in cell death.
Ferroptosis can be inhibited by various small molecules, including Ferrostatin-1 and Deferoxamine mesylate. Ferrostatin-1, an antioxidant, inhibits cell ferroptosis by inducing the disappearance of lipid hydroperoxides through the generation of an anti-ferroptotic effect. Deferoxamine mesylate, on the other hand, is an iron-chelating agent that binds to iron ions, reducing the accumulation and deposition of iron in tissues and suppressing cell ferroptosis.
Molecular Weight:
656.79
Pathway:
; Autophagy; Neuroscience; Cell Cycle/Checkpoint; Apoptosis
Purity:
0.9945
Research Area:
Tumour; Oesophageal Cancer; Tumour Immunology; Diabetes; Kidney Disease; Pathobiology; Epidemiology; Immunology; Research on molecular mechanisms
SMILES:
CS(O)(=O)=O.CC(=O)N(O)CCCCCNC(=O)CCC(=O)N(O)CCCCCNC(=O)CCC(=O)N(O)CCCCCN
Target:
Mitophagy; Beta Amyloid; Ferroptosis; Autophagy
References
1. Wang G, et al. In vitro assessment of deferoxamine on mesenchymal stromal cells from tumor and bone marrow. Environ Toxicol Pharmacol. 2017 Jan;49:58-64.
10. Wang S, Li F, Qiao R, et al. Arginine-Rich Manganese Silicate Nanobubbles as a Ferroptosis-Inducing Agent for Tumor-Targeted Theranostics[J]. ACS nano. 2018 Dec 26;12(12):12380-12392.
2. Sandau KB, et al. Regulation of the hypoxia-inducible factor 1alpha by the inflammatory mediators nitric oxide and tumor necrosis factor-alpha in contrast to desferroxamine and phenylarsine oxide. J Biol Chem. 2001 Oct 26;276(43):39805-11.
3. Wahl EA, et al. VEGF released by deferoxamine preconditioned mesenchymal stem cells seeded on collagen-GAG substrates enhances neovascularization. Sci Rep. 2016 Nov 10;6:36879.
4. Li Y, et al. Effects of deferoxamine on blood-brain barrier disruption after subarachnoid hemorrhage. PLoS One. 2017 Mar 1;12(3):e0172784.
5. Fine JM, et al. Intranasal deferoxamine engages multiple pathways to decrease memory loss in the APP/PS1 model of amyloid accumulation. Neurosci Lett. 2015 Jan 1;584:362-7.
6. Duscher D, et al. Comparison of the Hydroxylase Inhibitor Dimethyloxalylglycine and the Iron Chelator Deferoxamine in Diabetic and Aged Wound Healing. Plast Reconstr Surg. 2017 Mar;139(3):695e-706e
8. Sang M, Luo R, Bai Y, et al. BHQ-Cyanine-Based ?Off?On? Long-Circulating Assembly as a Ferroptosis Amplifier for Cancer Treatment: A Lipid-Peroxidation Burst Device[J]. ACS applied materials & interfaces. 2019, 11(46): 42873-42884.
9. Sang M, Luo R, Bai Y, et al. BHQ-Cyanine Based ?Off-On? Long-Circulating Assembly as Ferroptosis Amplifier for Cancer Treatment: a Lipid-Peroxidation Burst Device[J]. ACS applied materials & interfaces. 2019.