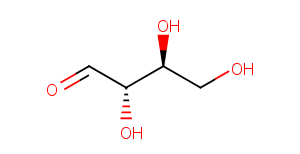
L-Erythrose
| Code | Size | Price |
|---|
| TAR-T20229-500mg | 500mg | £483.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
cool pack
Storage:
-20℃
Images
Documents
Further Information
Bioactivity:
L(+)-Erythrose is an aldotetrose rare sugar. It may be used in glycation studies and as a reference compound in tetrose carbohydrate separation and quantitation analysis. It also can be used to help identify and characterize erythrose reductase(s) and to study the mechanisms of mutarotation in mono sugars.
CAS:
533-49-3
Formula:
C4H8O4
Molecular Weight:
120.104
Purity:
0.98
SMILES:
OC[C@H](O)[C@H](O)C=O
References
1. Draskovits M, Stanetty C, Baxendale IR, Mihovilovic MD. Indium- and Zinc-Mediated Acyloxyallylation of Protected and Unprotected Aldotetroses-Revealing a Pronounced Diastereodivergence and a Fundamental Difference in the Performance of the Mediating Metal. J Org Chem. 2018 Mar 2;83(5):2647-2659. doi: 10.1021/acs.joc.7b03063. Epub 2018 Feb 9. PubMed PMID: 29369620; PubMed Central PMCID: PMC5838623.
2. Zou X, Lin J, Mao X, Zhao S, Ren Y. Biosynthesis of L-Erythrose by Assembly of Two Key Enzymes in Gluconobacter oxydans. J Agric Food Chem. 2017 Sep 6;65(35):7721-7725. doi: 10.1021/acs.jafc.7b02201. Epub 2017 Aug 24. PubMed PMID: 28707464.
3. Zweckmair T, B?hmdorfer S, Bogolitsyna A, Rosenau T, Potthast A, Novalin S. Accurate analysis of formose reaction products by LC-UV: an analytical challenge. J Chromatogr Sci. 2014 Feb;52(2):169-75. doi: 10.1093/chromsci/bmt004. Epub 2013 Feb 1. PubMed PMID: 23377653.
4. Jasi?ski M, Lentz D, Reissig HU. Carbohydrate-auxiliary assisted preparation of enantiopure 1,2-oxazine derivatives and aminopolyols. Beilstein J Org Chem. 2012;8:662-74. Epub 2012 Apr 30. PubMed PMID: 23015812; PubMed Central PMCID: PMC3388852.