Lipodisq Control Sterile Solution
| Code | Size | Price |
|---|
| IAX-700-100-L001 | 1 ml | £197.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
Short term: +4°C. Long term: +4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Detergent-free Nano-formulation made of Styrene-maleic Acid Lipid Particles (SMALP)
Appearance:
Colourless clear aqueous solution.
Biological Activity:
Cell culture tested (human macrophage cell line) (MTT).
Recommended starting dilution: 1:200 or higher.
Optimal working concentrations depend on the applications and need to be determined.
Published procedures using Lipodisq formulations (Curcumin and IAXO TLR4 antagonists) in vivo rodent models at 3-10mg/kg.
Recommended route of administration is subcutaneous (s.c.) with oral or nasal application as a possible alternative, which needs to be optimised.
EClass:
32160000
Form (Short):
liquid
Formulation:
Liquid, detergent-free discoidal nano-formulation made of styrene-maleic acid lipid particles (SMALP), lecithin and sterile water.
Handling Advice:
Keep sterile. Avoid skin and eye contact.
Long Description:
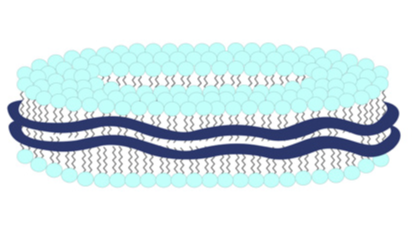
Chemical. A nanoparticle (11-40nm) drug delivery system comprising a discoidal phospholipid bilayer membrane stabilized by a chaperone molecule annulus. Internal properties of the phospholipid membrane support the disposition and stabilization of drug molecule candidates and preserve the native conformation of membrane molecules. The resulting encapsulated actives are rendered water-soluble and specialized for intra-cellular penetration/delivery via endosomal uptake mechanisms. Lipodisq™ solutions show a good safety profile and are suitable for in vitro and in vivo investigations.
Other data:
Lipodisq™ technology is covered by one or more of the following patents owned by Malvern Cosmeceutics Limited: AU2006253886, CA2611144, CN101184473B, EP1890675, GB2426703, IN261468, JP5142898, US8623414 and WO/2021/005340A1 pending. The purchaser is licensed under those patents to use these assemblies for the purpose of research and development only, but not for the purpose of delivery of agents for clinical use to humans or veterinary use to animals for therapeutic, diagnostic or prophylactic purposes, which uses are specifically prohibited.
Package Type:
Vial
Product Description:
A nanoparticle (11-40nm) drug delivery system comprising a discoidal phospholipid bilayer membrane stabilized by a chaperone molecule annulus. Internal properties of the phospholipid membrane support the disposition and stabilization of drug molecule candidates and preserve the native conformation of membrane molecules. The resulting encapsulated actives are rendered water-soluble and specialized for intra-cellular penetration/delivery via endosomal uptake mechanisms. Lipodisq™ solutions show a good safety profile and are suitable for in vitro and in vivo investigations.
Solubility Chemicals:
Soluble in water, PBS, Tris and other physiological solutions as formulated in a proprietary, thermostable, aqueous lipid nanoparticulate formulation (Lipodisq).
Transportation:
Non-Hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at +4°C.
References
Characterizing the structure of lipodisq nanoparticles for membrane protein spectroscopic studies: R. Zhang, et al.; Biochim. Biophys. Acta. 1848, 329 (2015) | Nano-size uni-lamellar lipodisq improved in situ auto-phosphorylation analysis of E. coli tyrosine kinase using (19)F nuclear magnetic resonance: D. Li, et al.; Protein Cell 6, 229 (2015) | Reconstitution of membrane proteins: a GPCR as an example: A.D. Goddard, et al.; Methods Enzymol. 556, 405 (2015) | The styrene?maleic acid copolymer: a versatile tool in membrane research: J.M. Doerr, et al.; Eur. Biophys. J. 45, 3 (2016) | From polymer chemistry to structural biology: The development of SMA and related amphipathic polymers for membrane protein extraction and solubilization: J.F. Bada Juarez, et al.; Chem. Phys. Lipids. 221, 167 (2019) | Effects of charged lipids on the physicochemical and biological properties of lipid?styrene maleic acid copolymer discoidal particles:M. Tanakaa, et al.; Biochim. Biophys. Acta. Biomembr. 1862, 183209 (2020) | Physicochemical Characterization, Toxicity and In Vivo Biodistribution Studies of a Discoidal, Lipid-Based Drug Delivery Vehicle: Lipodisq Nanoparticles Containing Doxorubicin: M.L. Torgersen, et al.; J. Biomed. Nanotechnol. 16, 41 (2020) | Understanding the Structural Pathways for Lipid Nanodisc Formation: How Styrene Maleic Acid Copolymers Induce Membrane Fracture and Disc Formation: V.A. Bjornestad, et al.; Langmuir 37, 6178 (2021) | Applications of Synthetic Polymer Discoidal Lipid Nanoparticles to Biomedical Research: M. Tanaka; Chem. Pharm. Bull. 70, 507 (2022) | Mechanisms of Formation, Structure, and Dynamics of Lipoprotein Discs Stabilized by Amphiphilic Copolymers: A Comprehensive Review: P.S. Orekhov, et al.; Nanomaterials 12, 361 (2022)