Metformin Lipodisq Sterile Solution
| Code | Size | Price |
|---|
| IAX-700-103-L001 | 1 ml | £197.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
Short term: +4°C. Long term: -20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
1,1-Dimethylbiguanide . HCl; NSC 91485; DMGG; LA 6023
Appearance:
Colourless clear aqueous solution
Biological Activity:
Discoidal nano-particles can incorporate hydrophobic, poorly water-soluble compounds, such as lipids, lipoproteins and glycolipids. - Cell culture tested (human macrophage cell line) (MTT). - Recommended starting dilution: 1:200 or higher. - Optimal working concentrations depend on the applications and need to be determined. - Published procedures using Lipodisq formulations (Curcumin and IAXO TLR4 antagonists) in vivo rodent models at 3-10mg/kg. Recommended route of administration is subcutaneous (s.c.) with oral or nasal application as a possible alternative, which needs to be optimized.
CAS:
1115-70-4
Concentration:
1mg/ml (0.1% w/vol)
EClass:
32160000
Form (Short):
liquid
Formulation:
Liquid, detergent-free discoidal nano-formulation made of styrene-maleic acid lipid particles (SMALP), lecithin and sterile water.
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry. Avoid skin and eye contact.
Hazards:
H302
InChi:
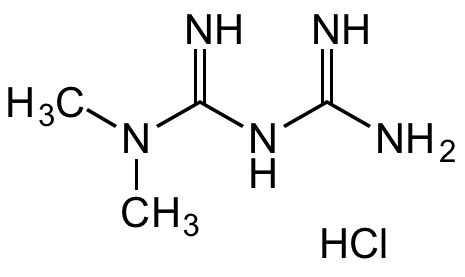
InChI=1S/C4H11N5.ClH/c1-9(2)4(7)8-3(5)6;/h1-2H3,(H5,5,6,7,8);1H
InChiKey:
OETHQSJEHLVLGH-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 1115-70-4. Formula: C4H11N5 . HCl. MW: 129.2 . 36.5. Metformin Lipodisq Sterile Solution is a ready-to-use nano-formulated aqueous solution. Metformin is an antihyperglycemic agent of the biguanide class, used for the management of type II diabetes and is currently prescribed to at least 120 million people worldwide. It is an AMPK activator and a mitochondrial electron transport chain complex I inhibitor, reducing mitochondrial reactive oxygen species (ROS). This antidiabetic and anti-hyperglycemic agent reduces blood glucose levels, improves insulin sensitivity, and decreases insulin resistance. Insulin sensitizer in non-alcoholic fatty liver disease (NAFLD). Increases plasma concentrations of the glucose-lowering gut incretin hormone glucagon-like peptide-1 (GLP-1), which may contribute to metformin?s glucose-lowering effect. An anticancer agent with antiproliferative and proapoptotic activity in cancer cell lines. Autophagy activator. Targets brown adipose tissue (BAT) in vivo and reduces oxygen consumption. Anti-inflammatory agent by inhibition of nuclear factor kappaB (NF-kappaB) via AMPK-dependent and independent pathways. Also described to inhibit NLRP3 inflammasome activation, subsequent caspase-1 cleavage and interleukin-1beta secretion. Since the emergence of SARS-CoV-2, Metformin has been investigated as a prophylactic agent for the prevention of COVID-19. Metformin Lipodisq is based on a nanoparticle (11-40nm) drug delivery system comprising a discoidal phospholipid bilayer membrane stabilized by a chaperone molecule annulus. Internal properties of the phospholipid membrane support the disposition and stabilization of drug molecule candidates and preserve the native conformation of membrane molecules. The resulting encapsulated actives are rendered water-soluble and specialized for intra-cellular penetration/delivery via endosomal uptake mechanisms. Lipodisq solutions show a good safety profile and are suitable for in vitro and in vivo investigations.
MDL:
MFCD00012582
Molecular Formula:
C4H11N5 . HCl
Molecular Weight:
129.2 . 36.5
Other data:
Lipodisq™ technology is covered by one or more of the following patents owned by Malvern Cosmeceutics Limited: AU2006253886, CA2611144, CN101184473B, EP1890675, GB2426703, IN261468, JP5142898, US8623414 and WO/2021/005340A1 pending. The purchaser is licensed under those patents to use these assemblies for the purpose of research and development only, but not for the purpose of delivery of agents for clinical use to humans or veterinary use to animals for therapeutic, diagnostic or prophylactic purposes, which uses are specifically prohibited.
Package Type:
Vial
Precautions:
P301+P312
Product Description:
Metformin Lipodisq Sterile Solution is a ready-to-use nano-formulated aqueous solution. Metformin is an antihyperglycemic agent of the biguanide class, used for the management of type II diabetes and is currently prescribed to at least 120 million people worldwide. It is an AMPK activator and a mitochondrial electron transport chain complex I inhibitor, reducing mitochondrial reactive oxygen species (ROS). This antidiabetic and anti-hyperglycemic agent reduces blood glucose levels, improves insulin sensitivity, and decreases insulin resistance. Insulin sensitizer in non-alcoholic fatty liver disease (NAFLD). Increases plasma concentrations of the glucose-lowering gut incretin hormone glucagon-like peptide-1 (GLP-1), which may contribute to metformin?s glucose-lowering effect. An anticancer agent with antiproliferative and proapoptotic activity in cancer cell lines. Autophagy activator. Targets brown adipose tissue (BAT) in vivo and reduces oxygen consumption. Anti-inflammatory agent by inhibition of nuclear factor kappaB (NF-kappaB) via AMPK-dependent and independent pathways. Also described to inhibit NLRP3 inflammasome activation, subsequent caspase-1 cleavage and interleukin-1beta secretion. Since the emergence of SARS-CoV-2, Metformin has been investigated as a prophylactic agent for the prevention of COVID-19. Metformin Lipodisq is based on a nanoparticle (11-40nm) drug delivery system comprising a discoidal phospholipid bilayer membrane stabilized by a chaperone molecule annulus. Internal properties of the phospholipid membrane support the disposition and stabilization of drug molecule candidates and preserve the native conformation of membrane molecules. The resulting encapsulated actives are rendered water-soluble and specialized for intra-cellular penetration/delivery via endosomal uptake mechanisms. Lipodisq solutions show a good safety profile and are suitable for in vitro and in vivo investigations.
Purity:
>95% (HPLC)
Signal Word:
Warning
SMILES:
CN(C(NC(N)=N)=N)C.Cl
Solubility Chemicals:
Soluble in water, PBS, Tris and other physiological solutions as formulated in a proprietary, thermostable, aqueous lipid nanoparticulate formulation (Lipodisq).
Transportation:
Non-Hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at +4°C.
References
Cellular and molecular mechanisms of metformin: an overview: B. Viollet, et al.; Clin. Sci. 122, 253 (2012) | Metformin in 2019: J. Flory & K. Lipska; JAMA 321, 1926 (2019) | Metformin Use Is Associated With Reduced Mortality in a Diverse Population With COVID-19 and Diabetes: A.B. Crouse, et al.; Front. Endocrinol. 11, 600439 (2021) | Metformin inhibition of mitochondrial ATP and DNA synthesis abrogates NLRP3 inflammasome activation and pulmonary inflammation: J. Flory & K. Lipska; Immunity 54, 1463 (2021) | Metformin in Patients With COVID-19: A Systematic Review and Meta-Analysis: Y. Li, et al.; Front. Med. 8, 704666 (2021) | Outpatient metformin use is associated with reduced severity of COVID-19 disease in adults with overweight or obesity: C.T. Bramante, et al.; J. Med. Virol. 93, 4273 (2021) | Metformin inhibition of mitochondrial ATP and DNA synthesis abrogates NLRP3 inflammasome activation and pulmonary inflammation: H. Xian, et al.; Immunity 54, 1463 (2021) | Metformin and Covid-19: Focused Review of Mechanisms and Current Literature Suggesting Benefit: S. Ibrahim, et al.; Front. Endocrinol. 12, 587801 (2021) | General References for Lipodisq™ Technology: | Responsive Hydrophobically Associating Polymers: A Review of Structure and Properties: S.R. Tonge & B.J. Tighe; Adv. Drug Deliv. Rev. 53, 109 (2001) | Detergent-free formation and physico-chemical characterization of nanosized lipidpolymer complexes: Lipodisq; M.C. Orwick, et al.; Angew. Chem. 51, 4653 (2012) | Physicochemical Characterization, Toxicity and In Vivo Biodistribution Studies of a Discoidal, Lipid-Based Drug Delivery Vehicle: Lipodisq Nanoparticles Containing Doxorubicin: M.L. Torgersen, et al.; J. Biomed. Nanotechnol. 16, 41 (2020) | Applications of Synthetic Polymer Discoidal Lipid Nanoparticles to Biomedical Research: M. Tanaka; Chem. Pharm. Bull. 70, 507 (2022) | Mechanisms of Formation, Structure, and Dynamics of Lipoprotein Discs Stabilized by Amphiphilic Copolymers: A Comprehensive Review: P.S. Orekhov, et al.; Nanomaterials 12, 361 (2022)