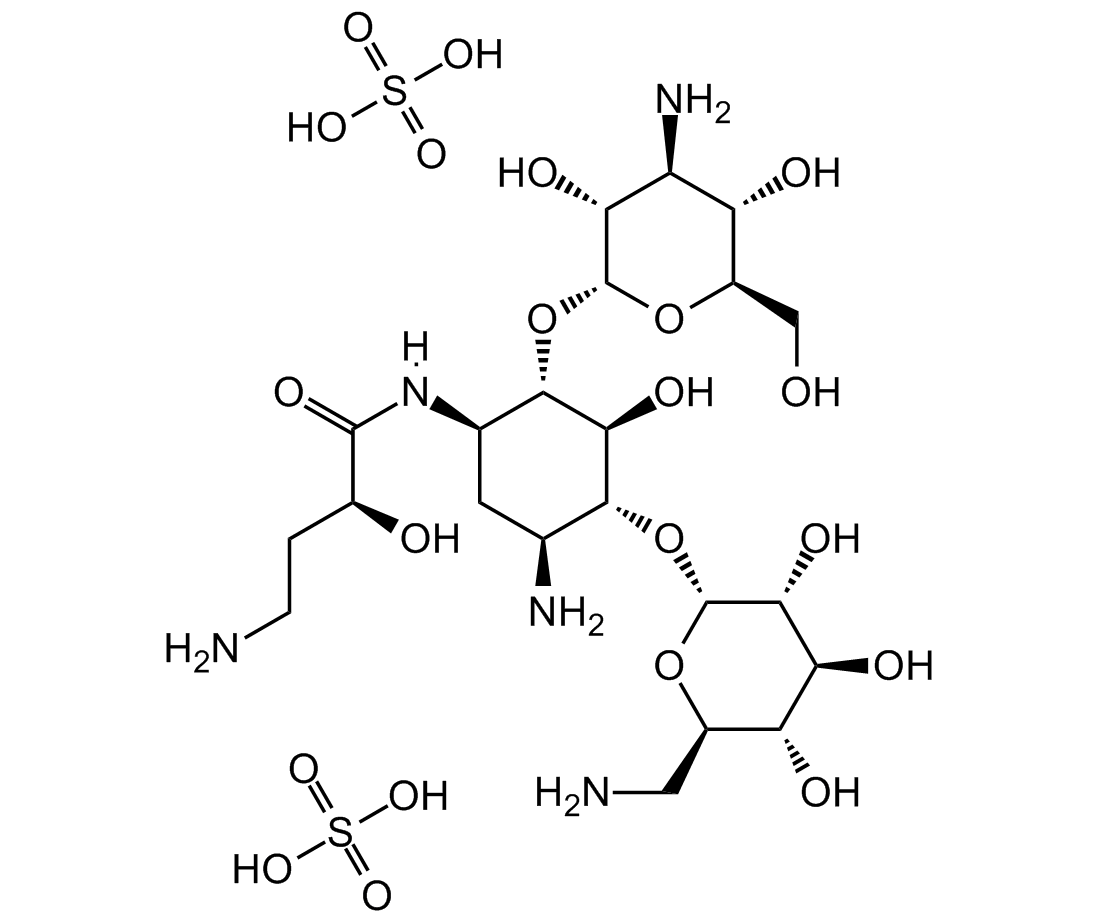
Amikacin disulfate salt
| Code | Size | Price |
|---|
| CDX-A0286-G001 | 1 g | £150.00 |
Quantity:
| CDX-A0286-G005 | 5 g | £428.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
Short term: +20°C. Long term: +4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Amikacin disulfate; Biodacyn; Selemycin; Amiglyde; BB-K 8; Novamin; Amikin
Appearance:
White to off-white powder.
CAS:
39831-55-5
EClass:
32160000
Form (Short):
solid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C22H43N5O13.2H2O4S/c23-2-1-8(29)20(36)27-7-3-6(25)18(39-22-16(34)15(33)13(31)9(4-24)37-22)17(35)19(7)40-21-14(32)11(26)12(30)10(5-28)38-21;2*1-5(2,3)4/h6-19,21-22,28-35H,1-5,23-26H2,(H,27,36);2*(H2,1,2,3,4)/t6-,7+,8-,9+,10+,11-,12+,13+,14+,15-,16+,17-,18+,19-,21+,22+;;/m0./s1
InChiKey:
FXKSEJFHKVNEFI-GCZBSULCSA-N
Long Description:
Chemical. CAS: 39831-55-5. Formula: C22H43N5O13 . 2H2SO4. MW: 781.76. Amikacin is a broad-spectrum aminoglycoside antibiotic and a semisynthetic analog of kanamycin. It irreversibly binds to 16S rRNA and the RNA-binding S12 protein of the 30S subunit and 50S subunit of prokaryotic ribosome and inhibits protein synthesis. It works in a concentration-dependent manner, and has better action in an alkaline environment. Amikacin disulfate is very active against most Gram-negative bacteria including gentamicin- and tobramycin-resistant strains, due to its resistance to inactivating enzymes. Amikacin disulfate also inhibits the infections caused by susceptible Nocardia and nontuberculous mycobacteria. Amikacin can be used to treat non-tubercular mycobacterial infections and tuberculosis when first-line drugs fail to control the infection.
MDL:
MFCD00167475
Molecular Formula:
C22H43N5O13 . 2H2SO4
Molecular Weight:
781.76
Package Type:
Vial
Product Description:
Amikacin is a broad-spectrum aminoglycoside antibiotic and a semisynthetic analog of kanamycin. It irreversibly binds to 16S rRNA and the RNA-binding S12 protein of the 30S subunit and 50S subunit of prokaryotic ribosome and inhibits protein synthesis. It works in a concentration-dependent manner, and has better action in an alkaline environment. Amikacin disulfate is very active against most Gram-negative bacteria including gentamicin- and tobramycin-resistant strains, due to its resistance to inactivating enzymes. Amikacin disulfate also inhibits the infections caused by susceptible Nocardia and nontuberculous mycobacteria. Amikacin can be used to treat non-tubercular mycobacterial infections and tuberculosis when first-line drugs fail to control the infection.
Product Line Areas NEW:
Biochemicals, Immunology
Product Type:
Chemical
Purity:
>97% (NMR)
SMILES:
OS(O)(=O)=O.OS(O)(=O)=O.O[C@@H]([C@@H]([C@H]1N)O[C@H]([C@@H]([C@H]2O)O)O[C@H](CN)[C@H]2O)[C@H]([C@@H](C1)N([H])C([C@H](CCN)O)=O)O[C@H]([C@@H]([C@H]3N)O)O[C@H](CO)[C@H]3O
Solubility Chemicals:
Soluble in water (50mg/ml).
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4A°C.
References
[1] H. Kawaguchi, et al.; J. Antibiot. 25, 695 (1972) | [2] P.K. Yu, et al.; Antimicrob. Agents Chemother. 4, 133 (1973) | [3] D. Zaske & K. Crossley; Minn. Med. 61, 123 (1978) (Review) | [4] F.D. Pien & P.W. Ho; Am. J. Hosp. Pharm. 38, 981 (1981) (Review) | [5] A.M. Ristuccia & B.A.Cunha; Ther. Drug Monit. 7, 12 (1985) (Review) | [6] M.S. Ramirez & M.E. Tolmasky; Molecules 22, 2267 (2017) (Review)