Suramin . sodium salt
| Code | Size | Price |
|---|
| AG-CR1-3575V-M050 | 50 mg | £60.00 |
Quantity:
| AG-CR1-3575V-M250 | 250 mg | £210.00 |
Quantity:
| AG-CR1-3575V-G001 | 1 g | £610.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
Short term storage:+4°C. Long term storage:+4°C.
Images
Documents
Further Information
Alternate Names/Synonyms:
Germanin; NSC 34936; SK 24728
Appearance:
White to off-white powder.
CAS:
129-46-4
EClass:
32160000
Form (Short):
solid
Handling Advice:
Keep cool and dry.
InChi:
InChI=1S/C51H40N6O23S6.6Na/c1-25-9-11-29(49(60)54-37-13-15-41(83(69,70)71)35-21-33(81(63,64)65)23-43(45(35)37)85(75,76)77)19-39(25)56-47(58)27-5-3-7-31(17-27)52-51(62)53-32-8-4-6-28(18-32)48(59)57-40-20-30(12-10-26(40)2)50(61)55-38-14-16-42(84(72,73)74)36-22-34(82(66,67)68)24-44(46(36)38)86(78,79)80;;;;;;/h3-24H,1-2H3,(H,54,60)(H,55,61)(H,56,58)(H,57,59)(H2,52,53,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80);;;;;;/q;6*+1/p-6
InChiKey:
VAPNKLKDKUDFHK-UHFFFAOYSA-H
Long Description:
Chemical. CAS: 129-46-4. Formula: C51H34N6O23S6 . 6Na. MW: 1291.2 . 137.9. Potent ATPase inhibitor [1]. Potent competitive inhibitor of reverse transcriptase. Shows anti-HIV activity [2, 3]. Anticancer compound [4, 5, 15]. Protein kinase C (PKC) inhibitor [4]. Potent inhibitor of melanoma heparanase and tumor cell metastasis [6]. Non-specific growth factors inhibitor (including PDGF, EGF, aFGF and bFGF) [7, 16]. TGF-beta1 inhibitor [8]. Topoisomerase I and II inhibitor [9]. Interleukin-1 (IL-1) inhibitor [10]. Interleukin-4 (IL-4) inhibitor [11]. G protein inhibitor [12]. P2X and P2Y purinergic receptor antagonist [13]. Antiangiogenic. Potent VEGF inhibitor [14, 15]. Telomerase inhibitor [17]. Shows adjuvant properties [18]. Regulates ryanodine receptor [19]. Direct adenylyl cyclase inhibitor [20]. Protein synthesis inhibitor [21]. SIRT1 (sirtuin 1) and SIRT5 (sirtuin 5) inhibitor [22, 23]. Immunosuppressive [24]. Antifibrotic agent [25]. Antiparasitic. Antiprotozoal. Athelmintic [26]. Cullin-RING E3 ubiquitin ligase inhibitor [29]. Inhibitor of the STING pathway via the inhibition of cGAMP synthase (cGAS) enzymatic activity [32]. Inhibits SARS-CoV-2 infection in cell culture by blocking early steps (binding/fusion) of the replication cycle. Potentially binds and inhibits nsp12 of SARS-CoV-2, binding to motifs harbouring the RNA-dependent RNA polymerases (RdRps) activity [34, 36]. Inhibits several dsDNA-binding proteins, including cGAS (inhibiting cGAS-STING and TLR9 mediated inflammatory responses), Mcm1040 and DNA topoisomerase II, and most recently also the AIM2 inflammasome. Effective inhibitor of dsDNA-induced inflammation [38].
MDL:
MFCD00210217
Molecular Formula:
C51H34N6O23S6 . 6Na
Molecular Weight:
1291.2 . 137.9
Other Data:
Water Content (by Karl Fischer): <15%
Sodium content: 8.0 - 9.5%
Package Type:
Vial
Product Description:
Potent ATPase inhibitor [1]. Potent competitive inhibitor of reverse transcriptase. Shows anti-HIV activity [2, 3]. Anticancer compound [4, 5, 15]. Protein kinase C (PKC) inhibitor [4]. Potent inhibitor of melanoma heparanase and tumor cell metastasis [6]. Non-specific growth factors inhibitor (including PDGF, EGF, aFGF and bFGF) [7, 16]. TGF-beta1 inhibitor [8]. Topoisomerase I and II inhibitor [9]. Interleukin-1 (IL-1) inhibitor [10]. Interleukin-4 (IL-4) inhibitor [11]. G protein inhibitor [12]. P2X and P2Y purinergic receptor antagonist [13]. Antiangiogenic. Potent VEGF inhibitor [14, 15]. Telomerase inhibitor [17]. Shows adjuvant properties [18]. Regulates ryanodine receptor [19]. Direct adenylyl cyclase inhibitor [20]. Protein synthesis inhibitor [21]. SIRT1 (sirtuin 1) and SIRT5 (sirtuin 5) inhibitor [22, 23]. Immunosuppressive [24]. Antifibrotic agent [25]. Antiparasitic. Antiprotozoal. Athelmintic [26]. Cullin-RING E3 ubiquitin ligase inhibitor [29]. Inhibitor of the STING pathway via the inhibition of cGAMP synthase (cGAS) enzymatic activity [32]. Inhibits SARS-CoV-2 infection in cell culture by blocking early steps (binding/fusion) of the replication cycle. Potentially binds and inhibits nsp12 of SARS-CoV-2, binding to motifs harbouring the RNA-dependent RNA polymerases (RdRps) activity [34, 36]. Inhibits several dsDNA-binding proteins, including cGAS (inhibiting cGAS-STING and TLR9 mediated inflammatory responses), Mcm1040 and DNA topoisomerase II, and most recently also the AIM2 inflammasome. Effective inhibitor of dsDNA-induced inflammation [38].
Purity:
>98% (HPLC).
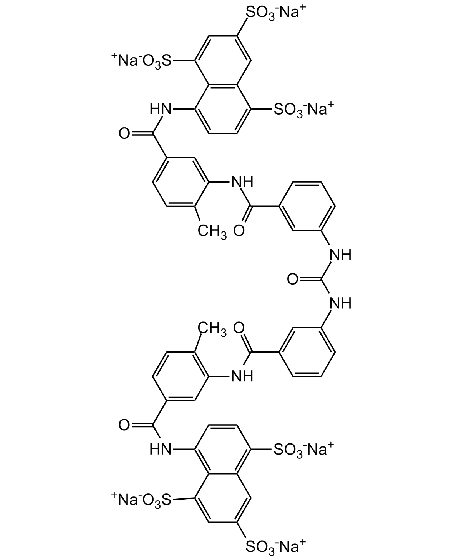
SMILES:
[Na+].[Na+].[Na+].[Na+].[Na+].[Na+].CC1=CC=C(C=C1NC(=O)C1=CC(NC(=O)NC2=CC=CC(=C2)C(=O)NC2=C(C)C=CC(=C2)C(=O)NC2=C3C(C=C(C=C3S([O-])(=O)=O)S([O-])(=O)=O)=C(C=C2)S([O-])(=O)=O)=CC=C1)C(=O)NC1=CC=C(C2=C1C(=CC(=C2)S([O-])(=O)=O)S([O-])(=O)=O)S([O-])(=O)=O
Solubility Chemicals:
Soluble in water (50mM) or DMSO (10mM). Sparingly soluble in ethanol.
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
Suramin: a potent ATPase inhibitor which acts on the inside surface of the sodium pump: P.A. Fortes, et al.; Biochim. Biophys. Acta 318, 262 (1973) | Suramin: a potent inhibitor of the reverse transcriptase of RNA tumor viruses: E. De Clerq; Cancer Lett. 8, 9 (1979) | Suramin protection of T cells in vitro against infectivity and cytopathic effect of HTLV-III: H. Mitsuya, et al.; Science 226, 172 (1984) | Suramin, an anti-cancer drug, inhibits protein kinase C and induces differentiation in neuroblastoma cell clone NB2A: C.E. Hensey, et al.; FEBS Lett. 258, 156 (1989) | Suramin: prototype of a new generation of antitumor compounds: R.V. La Rocca, et al.; Cancer Cells 2, 106 (1990) (Review) | Suramin. A potent inhibitor of melanoma heparanase and invasion: M. Nakajima, et al.; J. Biol. Chem. 266, 9661 (1991) | Nature of the interaction of growth factors with suramin: C.R. Middaugh, et al.; Biochemistry 31, 9016 (1992) | The antiproliferative effect of suramin on the cancer cell line SW-13 is mediated by the inhibition of transforming growth factor beta 1 (TGF-beta 1): R. Danesi, et al.; Pharmacol. Res. 25, 17 (1992) | Suramin inhibits DNA damage in human prostate cancer cells treated with topoisomerase inhibitors in vitro: H. Yamazaki, et al.; Prostate 23, 25 (1993) | Suramin blocks the binding of interleukin-1 to its receptor and neutralizes IL-1 biological activities: G. Strassmann, et al.; Int. J. Immunopharmacol. 16, 931 (1994) | Suramin blocks binding of interleukin-4 to its receptors on human tumor cells and interleukin-4-induced mitogenic response: P. Leland, et al.; Oncol. Res. 7, 227 (1995) | Suramin analogues as subtype-selective G protein inhibitors: M. Freissmuth, et al.;~Mol. Pharmacol. 49, 602 (1996) | PPADS and suramin as antagonists at cloned P2Y- and P2U-purinoceptors: S.J. Charlton, et al.; Br. J. Pharmacol. 118, 704 (1996) | Suramin is a potent inhibitor of vascular endothelial growth factor. A contribution to the molecular basis of its antiangiogenic action: J. Waltenberger, et al.; J. Mol. Cell Cardiol. 28, 1523 (1996) | Antiangiogenic and antiproliferative activity of suramin analogues: A.R. Gagliardi, et al.; Cancer Chemother. Pharmacol. 41, 117 (1998) | Suppression of myocardial inflammation using suramin, a growth factor blocker: T. Shiono, et al.;~Circ. J. 66, 385 (2002) | Suramin suppresses growth, alkaline-phosphatase and telomerase activity of human osteosarcoma cells in vitro: K. Trieb & H. Blahovec; Int. J. Biochem. Cell Biol. 35, 1066 (2003) | Suramin has adjuvant properties and promotes expansion of antigen-specific Th1 and Th2 cells in vivo: M. Denkinger, et al.; Int. Immunopharmacol. 4, 15 (2004) | Functional regulation of the cardiac ryanodine receptor by suramin and calmodulin involves multiple binding sites: A.P. Hill, et al; Mol. Pharmacol. 65, 1258 (2004) | Modulation of adenylyl cyclase activity in young and adult rat brain cortex. Identification of suramin as a direct inhibitor of adenylyl cyclase: J. St?hr, et al.; J. Cell Mol. Med. 9, 940 (2005) | Inhibition by suramin of protein synthesis in vitro. Ribosomes as the target of the drug: M. Brigotti, et al.;~Biochimie 88, 497 (2006) | Structure-activity studies on suramin analogues as inhibitors of NAD+-dependent histone deacetylases (sirtuins): J. Trapp, et al.; ChemMedChem 2, 1419 (2007) | Structural basis of inhibition of the human NAD+-dependent deacetylase SIRT5 by suramin: A. Schuetz, et al.; Structure 15, 377 (2007) | Suramin inhibits the CD40-CD154 costimulatory interaction: a possible mechanism for immunosuppressive effects: E. Margolles-Clark, et al.; Biochem. Pharmacol. 77, 1236 (2009) | Tissue protective and anti-fibrotic actions of suramin: new uses of an old drug: N. Liu & S. Zhuang; Curr. Clin. Pharmacol. 6, 137 (2011) (Review) | The trypanocidal drug suramin and other trypan blue mimetics are inhibitors of pyruvate kinases and bind to the adenosine site: H.P. Morgan, et al.; J. Biol. Chem. 286, 31232 (2011) | Neutralization of Apis mellifera bee venom activities by suramin: C.Z. El-Kik, et al.; Toxicon 67, 55 (2013) | Suramin inhibits helicase activity of NS3 protein of dengue virus in a fluorescence-based high throughput assay format: C. Basavannacharya & S.G. Vasudevan;~BBRC 453, 539 (2014) | Suramin inhibits cullin-RING E3 ubiquitin ligases: K. Wu, et al.; PNAS 113, E2011 (2016) | Suramin is a potent inhibitor of Chikungunya and Ebola virus cell entry: L. Henss, et al.; Virol. J. 13, 149 (2016) | Suramin is a novel competitive antagonist selective to alpha1beta2gamma2 GABAA over rho1 GABAC receptors: H. Luo, et al.; Neuropharmacol. 141, 148 (2018) | Suramin potently inhibits cGAMP synthase, cGAS, in THP1 cells to modulate IFN-beta levels: M. Wang, et al.; Future Med. Chem. 10, 1301 (2018) | Evaluating the impact of suramin additive on CHO cells producing Fc-fusion protein: J.-H. Lim, et al.; Biotechnol. Lett. 41, 1255 (2019) | Suramin inhibits SARS-CoV-2 infection in cell culture by interfering 2 with early steps of the replication cycle: C. Salgado, et al.; Antimicrob. Agents Chemother. 64, e00900 (2020) | 100 Years of Suramin: N. Wiedemar, et al.; Antimicr. Agents Chemother. 64, e01168-19 (2020) (Review) | Suramin, Penciclovir and Anidulafungin bind nsp12, which governs the RNA-dependent-RNA polymerase activity of SARS-CoV-2, with higher interaction energy than Remdesivir, indicating potential in the treatment of Covid-19 infection: S.K. Dey, et al.; J. Biomol. Struct. Dyn. 40, 14067 (2022) | High Throughput Screening Targeting the Dengue NS3-NS5 Interface Identifies Antivirals against Dengue, Zika and West Nile Viruses: S.N.Y. Yang, et al.; Cells 11, 730 (2022) | Discovery of an inhibitor of DNA-driven inflammation that preferentially targets the AIM2 inflammasome: J.P. Green, et al.; iScience 26, 106758 (2023)