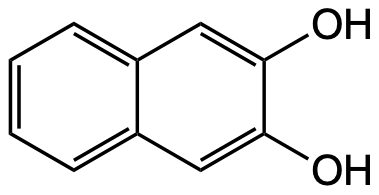
2,3-Dihydroxy-naphthalin
| Code | Size | Price |
|---|
| CDX-D0892-G100 | 100 g | £115.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
Short term: +20°C, Long term: +20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
2,3-DHN; 2,3-Naphthalenediol; 2,3-Dihydroxy-naphthalin; 2,3-Naphthalindiol
Appearance:
White to faint beige powder or crystals.
CAS:
92-44-4
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS05, GHS07
Handling Advice:
Keep under inert gas. Very hygroscopic.
Hazards:
H302 - H318
InChi:
InChi=1S/C10H8O2/c11-9-5-7-3-1-2-4-8(7)6-10(9)12/h1-6,11-12H
InChiKey:
JRNGUTKWMSBIBF-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 92-44-4. Formula: C10H8O2. MW: 160.17. 2,3-Dihydroxynaphthalene (2,3-DHN) is a naturally occurring catechol. 2,3-Dihydroxynaphthalene (2,3-DHN) can be used as a building block, starting material or reagent in organic synthesis to create more complex chemical compounds. The hydroxyl groups in 2,3-DHN can undergo various chemical reactions, such as esterification, oxidation, or substitution reactions, to produce a wide range of derivatives. 2,3-Dihydroxynaphthalene displays the ability to function as either an electron donor or acceptor in redox reactions. It also serves as an electron transfer agent in enzymatic reactions. 2,3-DHN and its derivatives can be used in the synthesis of dyes and pigments. 2,3-DHN and its derivatives have been shown to inhibit certain enzymes and have potential anti-tumor properties. 2,3-DHN is a metal chelators.
MDL:
MFCD00004073
Molecular Formula:
C10H8O2
Molecular Weight:
160.17
Package Type:
Vial
Precautions:
P264 - P270 - P280 - P301 + P312 - P305 + P351 + P338 - P501
Product Description:
2,3-Dihydroxynaphthalene (2,3-DHN) is a naturally occurring catechol. 2,3-Dihydroxynaphthalene (2,3-DHN) can be used as a building block, starting material or reagent in organic synthesis to create more complex chemical compounds. The hydroxyl groups in 2,3-DHN can undergo various chemical reactions, such as esterification, oxidation, or substitution reactions, to produce a wide range of derivatives. 2,3-Dihydroxynaphthalene displays the ability to function as either an electron donor or acceptor in redox reactions. It also serves as an electron transfer agent in enzymatic reactions. 2,3-DHN and its derivatives can be used in the synthesis of dyes and pigments. 2,3-DHN and its derivatives have been shown to inhibit certain enzymes and have potential anti-tumor properties. 2,3-DHN is a metal chelators.
Purity:
>98% (HPLC)
Signal Word:
Danger
SMILES:
Oc1cc2ccccc2cc1O
Solubility Chemicals:
Soluble in DMSO or methanol (1mg/ml).
Transportation:
Non-hazardous
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at RT.
References
(1) J.C. DiNardo, et al.; Toxicol. Appl. Pharmacol. 78, 163 (1985) | (2) P.K. Tarafder, et al.; Talanta 41, 1345 (1994) | (3) R. Shiman, et al.; J. Cell Biol. 236, 24637 (1994) | (4) S. Bhownik & U. Maitra; Chem. Commun. 48, 4624 (2012) | (5) J. Feng, et al.; Dalton Trans. 41, 8697 (2012) | (6) L.M. Laglera, et al.; Anal. Chem. 85, 2486 (2013) | (7) M. Burton, et al.; Bioorg. Med. Chem. 26, 4841 (2018)