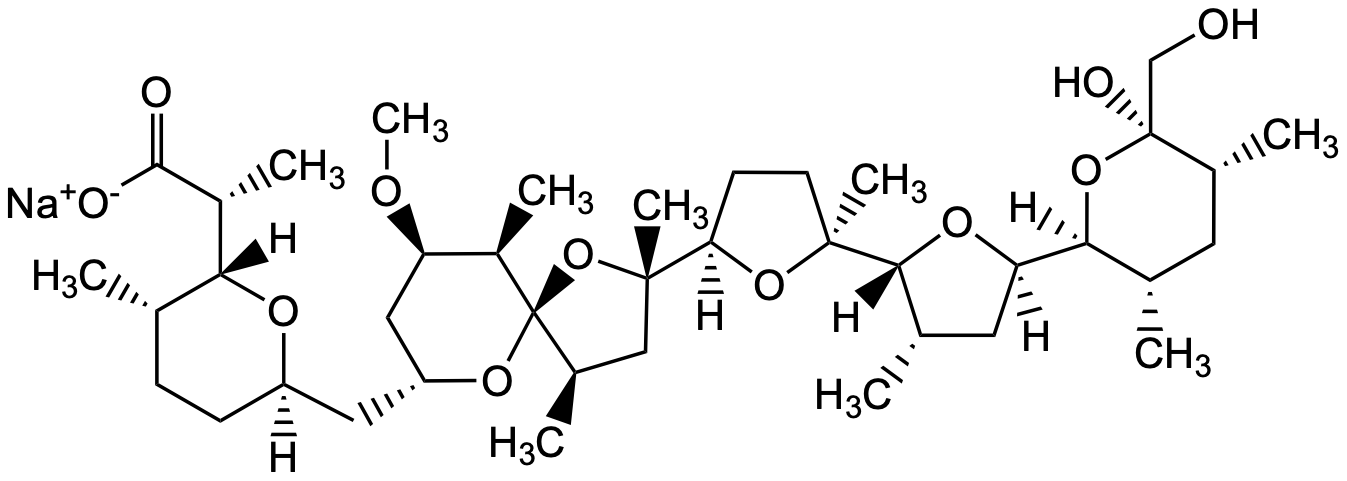
Nigericin sodium salt
| Code | Size | Price |
|---|
| CDX-N0094-M005 | 5 mg | £80.00 |
Quantity:
| CDX-N0094-M010 | 10 mg | £132.00 |
Quantity:
| CDX-N0094-M050 | 50 mg | £451.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
Short term: +20°C, Long term: +4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Antibiotic X464; Azalomycin M; Polyetherin A; Antibiotic K178; Helexin C
Appearance:
White to yellow powder.
CAS:
28643-80-3
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS06
Handling Advice:
Protect from light and moisture.
Hazards:
H301 - H319
InChi:
InChi=1S/C40H68O11.Na/c1-21-11-12-28(46-33(21)26(6)36(42)43)17-29-18-30(45-10)27(7)40(48-29)25(5)19-38(9,51-40)32-13-14-37(8,49-32)35-23(3)16-31(47-35)34-22(2)15-24(4)39(44,20-41)50-34;/h21-35,41,44H,11-20H2,1-10H3,(H,42,43);/q;+1/p-1/t21-,22-,23-,24+,25+,26+,27+,28+,29+,30+,31+,32+,33+,34-,35+,37-,38-,39-,40+;/m0./s1
InChiKey:
MOYOTUKECQMGHE-PDEFJWSRSA-M
Long Description:
Chemical. CAS: 28643-80-3. Formula: C40H67NaO11. MW: 746.94. Nigericin is an antibiotic, used as a high affinity ionophore for monovalent cations such as H+, K+, Na+, Pb2+. It shows antibacterial (Gram-positive), antifungal, antitumor and antiviral activity and disrupts membrane potential of mitochondria. It sprimary use is as a NLRP3 activator in inflammasome research.
MDL:
MFCD30377206
Molecular Formula:
C40H67NaO11
Molecular Weight:
746.94
Package Type:
Vial
PG:
III
Precautions:
P264 - P270 - P280 - P301 + P310 - P305 + P351 + P338 - P337 + P313
Product Description:
Nigericin is an antibiotic, used as a high affinity ionophore for monovalent cations such as H+, K+, Na+, Pb2+. It shows antibacterial (Gram-positive), antifungal, antitumor and antiviral activity and disrupts membrane potential of mitochondria. It sprimary use is as a NLRP3 activator in inflammasome research.
Purity:
>98% (TLC)
Signal Word:
Danger
SMILES:
[Na+].[H][C@@]1(CC[C@H](C)[C@@]([H])(O1)[C@@H](C)C([O-])=O)C[C@]2([H])C[C@@H](OC)[C@@H](C)[C@]3(O2)O[C@@](C)(C[C@H]3C)[C@@]4([H])CC[C@](C)(O4)[C@]5([H])O[C@]([H])(C[C@@H]5C)[C@@]6([H])O[C@@](O)(CO)[C@H](C)C[C@@H]6C
Solubility Chemicals:
Soluble in methanol or ethanol. Insoluble in water.
Source / Host:
Synthetic
Transportation:
Excepted Quantity
UN Nummer:
UN3462
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
(1) R. L. Harned, et al.; Antibiot. Chemother. 1, 594 (1951) | (2) S. Estrada-O, et al.; J. Biol. Chem. 242, 2925 (1967) | (3) D. Perregaux, et al.; J. Immunol. 149, 1294 (1992) | (4) B. Baibakov, et al.; Int. J. Oncol. 3, 1127 (1993) | (5) N. Watanabe, et al.; Cytokine 10, 645 (1998) | (6) S. Mariathasan, et al.; Nature 440, 228 (2006) | (7) C. Wang, et al.; Sci. Immunol. 6, eabj3859 (2021)