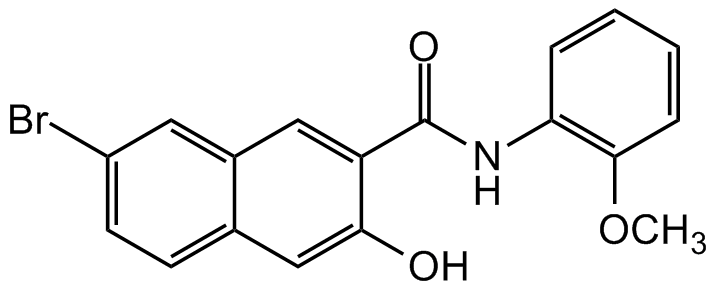
Naphthol AS-BI
| Code | Size | Price |
|---|
| CDX-N0239-G001 | 1 g | £161.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
Short term: +4°C, Long term: -20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
7-Bromo-3-hydroxy-N-(2-methoxyphenyl)-2-naphthamide; NSC 367089; C.I. 37566; C.I. Azoic Coupling Component 45
Appearance:
White to off-white powder.
CAS:
1237-75-8
EClass:
32160000
Form (Short):
solid
Handling Advice:
Keep under inert gas.Very hygroscopic.
InChi:
InChI=1S/C18H14BrNO3/c1-23-17-5-3-2-4-15(17)20-18(22)14-9-12-8-13(19)7-6-11(12)10-16(14)21/h2-10,21H,1H3,(H,20,22)
InChiKey:
JIEINYQEXWLMCU-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 1237-75-8. Formula: C18H14BrNO3. MW: BD9837. Naphthol AS-BI is a fluorescent dye commonly used as a building block or intermediate to produce fluorigenic substrates or as a coupling reagent to produce azo dyes. After cleavage of the fluorigenic substrates by esterases the free Naphthol AS-BI can couple with diazonium salts to form different types of fluorescent azo dyes.
MDL:
MFCD00004077
Molecular Formula:
C18H14BrNO3
Molecular Weight:
372.21
Package Type:
Vial
Product Description:
Naphthol AS-BI is a fluorescent dye commonly used as a building block or intermediate to produce fluorigenic substrates or as a coupling reagent to produce azo dyes. After cleavage of the fluorigenic substrates by esterases the free Naphthol AS-BI can couple with diazonium salts to form different types of fluorescent azo dyes.
Purity:
>99% (HPLC)
SMILES:
O=C(NC1=C(OC)C=CC=C1)C2=CC3=CC(Br)=CC=C3C=C2O
Solubility Chemicals:
Soluble in DMF (100mg/ml).
Source / Host:
Synthetic
Transportation:
Non-hazardous
UNSPSC Number:
41105331
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) D.C. Livingston, et al.; Histochemie 24, 159 (1970) | (2) P.K. Sinha & R. Gossrau; Histochem. J. 16, 334 (1984) | (3) D.E. Mahan, et al.; Anal. Biochem. 162, 163 (1987) | (4) G. Lu, et al.; J. Org. Chem. 71, 1769 (2006)