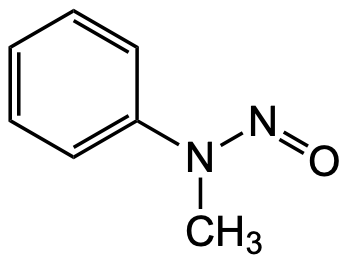
N-Nitrosomethylphenylamine (NMPA)
| Code | Size | Price |
|---|
| CDX-N0372-G001 | 1 g | £92.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
Short term: +4°C, Long term: -20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
NMPA; N-Methyl-N-nitrosoaniline; N-Methyl-N-nitrosobenzenamine; N-Methyl-N-phenylnitrosamine; NSC137
Appearance:
Orange liquid.
CAS:
614-00-6
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS02, GHS06, GHS08
Handling Advice:
Protect from light and moisture.
Hazards:
H225 - H301 + H311 + H331 - H370
InChi:
InChi=1S/C7H8N2O/c1-9(8-10)7-5-3-2-4-6-7/h2-6H,1H3
InChiKey:
MAXCWSIJKVASQC-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 614-00-6. Formula: C7H8N2O. MW: 136.15. N-nitrosomethylphenylamine (NMPA) is a nitrosamine and is known to be a potent carcinogen associated with cancer development in animals and humans. NMPA can damage DNA and cause mutations in genes that control cell growth and division. It reacts with DNA in vitro to form genotoxic activity, which may lead to cell death or mutagenesis. NMPA is formed in various industrial processes, including the synthesis of certain chemicals. It serves as a building block in the synthesis of diverse compounds and functions as an analytical reagent for environmental monitoring. This compound can be used as analytical reference material. NMPA is useful for studying enzymatic denitrosation processes. NMPA could be used as a tumor initiator, as a reagent for preparing carcinogenic animal diseases model.
MDL:
MFCD00045675
Molecular Formula:
C7H8N2O
Molecular Weight:
136.15
Package Type:
Vial
PG:
III
Precautions:
P210 - P233 - P280 - P301 + P310 - P303 + P361 + P353 - P304 + P340 + P311
Product Description:
N-nitrosomethylphenylamine (NMPA) is a nitrosamine and is known to be a potent carcinogen associated with cancer development in animals and humans. NMPA can damage DNA and cause mutations in genes that control cell growth and division. It reacts with DNA in vitro to form genotoxic activity, which may lead to cell death or mutagenesis. NMPA is formed in various industrial processes, including the synthesis of certain chemicals. It serves as a building block in the synthesis of diverse compounds and functions as an analytical reagent for environmental monitoring. This compound can be used as analytical reference material. NMPA is useful for studying enzymatic denitrosation processes. NMPA could be used as a tumor initiator, as a reagent for preparing carcinogenic animal diseases model.
Purity:
>98% (NMR)
Signal Word:
Danger
SMILES:
O=NN(C)C1=CC=CC=C1
Solubility Chemicals:
Slightly soluble in DMSO, methanol, ethanol or chloroform.
Source / Host:
Synthetic
Transportation:
Excepted Quantity
UN Nummer:
UN2810
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) C.M. Goodall, et al.; Toxicol. Appl. Pharmacol. 17, 426 (1970) | (2) W. Lijinsky & R.M. Kovatch; Cancer Res. 48, 6648 (1988) | (3) S.R. Koepke, et al.; IARC Sci. Publ. 105, 346 (1991) | (4) T. Scheper, et al.; Chem. Biol. Interact. 77, 81 (1991) | (5) M. Stiborova, et al.; Cancer Lett. 110, 11 (1996) | (6) M. Stiborova, et al.; Cancer Lett. 138, 61 (1999) | (7) Y. Li & S.S. Hecht; Int. J. Mol. Sci. 23, 4559 (2022) | (8) M. Bignami, et al.; Efsa J. 21, e07884 (2023)