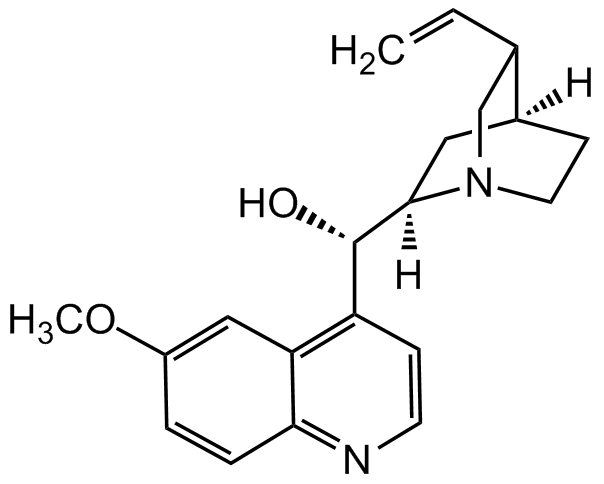
Quinidine anhydrous
| Code | Size | Price |
|---|
| CDX-Q0029-G010 | 10 g | £68.00 |
Quantity:
| CDX-Q0029-G050 | 50 g | £242.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Host Type: Plant
Regulatory Status: RUO
Shipping:
Ambient
Storage:
Short term: +20°C, Long term: +20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
alpha-(6-Methoxy-4-quinolyl)-5-vinyl-2-quinuclidinemethanol
Appearance:
White to faint yellow powder.
CAS:
56-54-2
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS06
Handling Advice:
Protect from light and moisture.
Hazards:
H301 - H317
InChi:
InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19+,20-/m0/s1
InChiKey:
LOUPRKONTZGTKE-LHHVKLHASA-N
Long Description:
Chemical. CAS: 56-54-2. Formula: C20H24N2O2. MW: BD9837. Quinidine is a dextrorotatory stereoisomer of Quinine with anti-malarial, anti-arrhythmic (class 1A), anti-inflammatory, anti-cancer and anti-viral properties. Quinidine acts as a blocker of voltage-gated sodium channels. Inhibition of the Nav1.5 channel is specifically involved in its anti-arrhythmic effects as a class I anti-arrhythmic agent. Quinidine also blocks certain voltage-gated potassium channels (e.g. Kv1.4, Kv4.2, hERG, among others), acts as an anti-muscarinic. Quinidine blocks alpha1-adrenoceptors (alpha1ARs) and is an inhibitor of cytochrome P450 and acetylcholinesterase.
MDL:
MFCD00135581
Molecular Formula:
C20H24N2O2
Molecular Weight:
324.42
Package Type:
Vial
PG:
III
Precautions:
P261 - P264 - P270 - P280 - P301 + P310 - P302 + P352
Product Description:
Quinidine is a dextrorotatory stereoisomer of Quinine with anti-malarial, anti-arrhythmic (class 1A), anti-inflammatory, anti-cancer and anti-viral properties. Quinidine acts as a blocker of voltage-gated sodium channels. Inhibition of the Nav1.5 channel is specifically involved in its anti-arrhythmic effects as a class I anti-arrhythmic agent. Quinidine also blocks certain voltage-gated potassium channels (e.g. Kv1.4, Kv4.2, hERG, among others), acts as an anti-muscarinic. Quinidine blocks alpha1-adrenoceptors (alpha1ARs) and is an inhibitor of cytochrome P450 and acetylcholinesterase.
Purity:
>99% (Titr.)
Signal Word:
Danger
SMILES:
O[C@@H](C1=C(C=C(OC)C=C2)C2=NC=C1)[C@]3([H])[N@](C[C@@H]4C=C)CC[C@@]4([H])C3
Solubility Chemicals:
Soluble in DMSO (20mg/ml) or ethanol (5mg/ml).
Source / Host:
Isolated from plant.
Transportation:
Excepted Quantity
UN Nummer:
UN2811
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at RT.
References
(1) N.J. White, et al.; Lancet 2, 1069 (1981) | (2) K. Schenck-Gustafsson, et al.; Am. J. Cardiol. 51, 777 (1983) | (3) L. Crevasse; Am. J. Cardiol. 62, 22I (1988) | (4) A Yatani, et al.; Circ. Res. 73, 351 (1993) | (5) J.A. Yao, et al.; J. Pharmacol. Exp. Ther. 279, 856 (1996) | (6) K. Shibata, et al.; Circulation 97, 1227 (1998) | (7) S. Wang, et al.; J. Physiol. 546, 387 (2003) | (8) J.M. Hutzler, et al.; Chem. Res. Toxicol. 16, 450 (2003) | (9) L.A. McLaughlin, et al.; J. Biol. Chem. 280, 38617 (2005) | (10) M.K. Pugsley, et al.; Clin. Exp. Pharmacol. Physiol. 32, 60 (2005) | (11) B. Bozic, et al.; Mini Rev. Med. Chem. 18, 468 (2018) | (12) L. Vitali Serdoz, et al.; Pharmacol. Res. 144, 257 (2019) | (13) Z. Che, et al.; Chem. Biodivers. 17, e1900696 (2020) | (14) Z. Li, et al.; Angew. Chem. Int. Ed. Engl. 60, 11474 (2021)